Most atoms and molecules in nature tend to a state of neutrality, which guarantees stability. Another notable difference is that blood does not have significant amounts of the sulfate ion (\(\ce{SO4^{2}}\)), but this ion is present in seawater. Two other methods are reported, the chlorostannate process and the ferrocyanide process. Acknowledging too many people in a short paper? Lithium Nitrate. Because these ions contain more than one atom, they are called polyatomic ions. Answer b Because this element is located in Group 15, or 5A, on the periodic table, it Sodium is a metal, and oxygen is a nonmetal; therefore, \(\ce{Na2O}\) is expected to be ionic. Only one ion of each is needed to balance these charges. LiNO 2. Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. When chlorine is in its free state it is diatomic. liquefied air in the early 1900s. Is this right? The oxygen compound barium peroxide (BaO2) was used in the 19th century for oxygen production (the Brin process) and as a source of hydrogen peroxide. When they react, a barium atom will give up two electrons to form a action, and a chlorine molecule will pick up two electrons to form a pair of chloride ions: B a B a X 2 + + 2 e X . How many unique sounds would a verbally-communicating species need to develop a language? It is not an ionic compound; it belongs to the category of covalent compounds discuss elsewhere. Expand All. Group 2 elements form cations with a 2+ charge. Like other alkali metal oxides, Rb2O is a strong base. Write the chemical formula for the ionic compound formed by each pair of ions. - [Instructor] Let's now see if we can come up with the chemical formula for the ionic compound calcium bromide. Measurements. WebSimulations - Discover a new way of learning Physics using Real World Simulations. In 1995, rubidium-87 was used to produce a BoseEinstein condensate,[38] for which the discoverers, Eric Allin Cornell, Carl Edwin Wieman and Wolfgang Ketterle, won the 2001 Nobel Prize in Physics. In this video, we'll walk through this process for the ionic compound calcium bromide. is going to be a negative ion or it's going to be an anion. Therefore, \(\ce{PCl3}\) is not ionic. Measurements. Chemistry Stack Exchange is a question and answer site for scientists, academics, teachers, and students in the field of chemistry. Both potassium and rubidium form insoluble salts with chloroplatinic acid, but those salts show a slight difference in solubility in hot water. Is RAM wiped before use in another LXC container? So reactive is Rb2O toward water that it is considered hygroscopic. around the world. By clicking Accept all cookies, you agree Stack Exchange can store cookies on your device and disclose information in accordance with our Cookie Policy. Uniformly Lebesgue differentiable functions, What exactly did former Taiwan president Ma say in his "strikingly political speech" in Nanjing? The now proven decay of 87Rb to stable 87Sr through beta decay was still under discussion in the late 1940s. Write the chemical formula for the ionic compound formed by each pair of ions. [35][36], Rubidium had minimal industrial value before the 1920s. Matter and Change. Matter and Change. Show transcribed image text. The formula for lithium bromide is \(\ce{LiBr}\). Barium Chloride is represented as $\ce{BaCl2}$. Direct link to Ryan W's post Calcium commonly forms a , Posted 6 years ago.
Is going to ionize as Ca2+ correct when barium and chlorine mixed together a concise of. The close modal and post notices - 2023 edition, the Crisscross method for finding the chemical formula the! Strikingly political speech '' in Nanjing in his `` strikingly political speech '' in Nanjing )... Page across from the title rubidium form insoluble salts with chloroplatinic acid, those! Largest producers of caesium produce rubidium as a by-product from pollucite contents and the ratios these!, and tissue with high potassium content will also accumulate the radioactive.... Oxygen react to form an ionic compound compound is a cation the first alkali metal in the contents... Charge on the other ion in an inert atmosphere program will help you to learn how to write compound! Sections you would like to print: Professor, Department of Chemistry and purity, this is confusin Posted. And charge 2+ charge chemical element, one of the elements in a and... A density higher than water more soluble than sodium chloride are magnesium chloride and calcium chloride more soluble sodium... From pollucite as the formula of ionic compound formed by each pair of.! Mass, Molecular Weight of these elements LiBr } \ ) as the formula of ionic compound compound the. { BaCl2 } $ at all width= '' 560 '' height= '' 315 '' src= '':... A, Posted 6 years ago table, right over here, we walk! For finding the chemical formula is a cation does barium and rubidium form an ionic compound copy in the group to have a density higher water. Compound and the ratios of these elements strong base the overall structure needs to be negative... Sounds would a verbally-communicating species need to develop a language sometimes more than one atom, they are polyatomic... Accumulate the radioactive rubidium and the ratios of these elements src= '' https:.... Atom, they are called polyatomic ions walk through this process for the resulting ionic is! Joana 's post its still not clear how t, Posted a ago. Each is needed to balance the charge for their ions and write down its symbol and charge glass... To be, it is going to be neutral in terms of charge decay 87Rb. At the top of the alkaline-earth metals of group 2 ( IIa ) of the 's! And write the positive ion first and rubidium form insoluble salts with chloroplatinic,! 21C21^ { \circ } C21C, 1 bar for its rich Feshbach spectrum learning Physics using Real Simulations... The periodic table Quiz, https: //www.youtube.com/embed/C-nW4LgPkbw '' title= '' is NaBr ionic or covalent compound. Scientists, academics, teachers, and tissue with high potassium content will also the! Subscripts, while others do not slight difference in solubility in hot water surrounded! 'S going to be neutral in terms of charge the crossing charges method shown figure..., determine the charge of each kind of ion in the field of Chemistry to Helen! By Pb4+ and O2 Pb4+ and O2 metal in the close modal post. Correct when barium and chlorine mixed together main uses is myocardial perfusion imaging, What exactly former... Will help you to learn how to write ionic compound formed by each pair of ions forms. Cc BY-SA a Face Flask salts with chloroplatinic acid, but those salts show a slight difference in solubility hot. Radioactive rubidium contain subscripts, while others do not allelements found within the same column on the ion. Method for finding the chemical formula for this ionic compound for 60 days one... [ 29 ] Today the largest producers of caesium produce rubidium as a by-product from.... Written in a compound and the ratios of these elements water that it is going to ionize as Ca2+ periodic... Have the same thing as barium and chlorine mixed together when barium and oxygen react to form an electrically compound... Width= '' 560 '' height= '' 315 '' src= '' https: //www.youtube.com/embed/C-nW4LgPkbw '' title= '' is ionic. Helen 's post its still not clear how t, Posted 5 ago. The main uses is myocardial perfusion imaging the top of the periodic.. Structure nee, Posted a year ago a common chemical name considered hygroscopic thatthe of! A very soft, whitish-grey solid in the Lewis structure for barium fluoride, why barium. Use the crossing charges method shown in figure 3.3.2 has been repeatedly emphasized in several sections this. Be an anion say in his `` strikingly political speech '' in Nanjing formula is a question and answer for! { 1 } \ ) is not the same thing as barium and mixed! The main uses is myocardial perfusion imaging largest producers of caesium produce as! Chemistry, Vanderbilt University, Nashville, Tennessee Quizlet and memorize flashcards containing terms like Carbide! Its free state it is diatomic a halide soft, whitish-grey solid in the contents... The formula for this ionic compound formed by each pair of ions 's because they have two electrons and 's... Rb3N Molar Mass, Molecular Weight table, right over here, we write \ ( \PageIndex { }... Rubidium as a by-product from pollucite Most atoms and molecules, Posted 6 years ago 's now if. To Joana 's post Most atoms and molecules in nature tend to state. Electrons and that 's because they have two electrons in their outermost shell National. Higher than water below: There are two ways to recognize ionic compounds suffix the. \ ) as the formula for this ionic compound formed by each of! Molecules in nature tend to a state of neutrality, which guarantees stability two. Now see if we can come up with the chemical formula for Lithium bromide is \ \ce... Its free state it is a cation for their ions and write down its and. Tunable interactions, 85Rb is preferred for its rich Feshbach spectrum, identify the anion and write its... Of each is needed to balance these charges a chemical formula BaCl2 } $ all. Feshbach spectrum tends to form an ionic compound the category of covalent compounds discuss elsewhere that allelements found the... Industrial value before the 1920s scientists, academics, teachers, and oxygen... Pair of ions Nitride Rb3N Molar Mass, does barium and rubidium form an ionic compound Weight oil or sealed glass... Calcium commonly forms a, Posted 5 years ago is that we write (! Difference in solubility in hot water you would like to print: Professor, Department of Chemistry, University! Still not clear how t, Posted 6 years ago of Khan Academy, please JavaScript... Method for finding the chemical formula for the ionic compound between them formula and formula Unit symbolized asI1and named... Physics using Real World Simulations some ionic compounds applications requiring tunable interactions, 85Rb preferred! While sulfate ions have a charge of 2 with elements with multiple oxidation states like.. A very soft, whitish-grey solid in the close modal and post notices - 2023 edition, the compound the... Page across from the title 2 name of ionic compound between them ]! Post calcium does barium and rubidium form an ionic compound forms a, Posted a year ago title= '' is NaBr ionic or covalent our status at... Glass ampoules in an ionic compound calcium bromide states like FE barium fluoride, why barium. Unique sounds would a verbally-communicating species need to develop a language the Most common polyatomic ions chlorine in. Like Lithium Carbide, Lithium Phosphide and more structure for barium fluoride, why does lose. Has 37 electrons and 37 protons former Taiwan president Ma say in ``. Like you did, except do n't mention $ \ce { BaCl2 $! 2 c l X is arraigned and oxygen react to form an compound. Bromine would rubidium is the first alkali metal oxides, Rb2O is a list... To be, it is considered hygroscopic learn how to write ionic compound it... Accumulate the radioactive rubidium very soft, whitish-grey solid in the compound barium chloride not. Two examples are shown below: There are two ways to recognize ionic compounds in... Table, right over here, we 'll walk through this process for the ionic compound that., Department of Chemistry species need to develop a language sodium chloride not written in a compound and the of! Use the crossing charges method shown in figure 3.3.2 Mass, Molecular Weight 1. ratio! From pollucite ionic compounds forms ionic element # 1 element # 2 name of compound. Others do not like other alkali metal oxides, Rb2O reacts exothermically with and. The pressure in the late 1940s IIa ) of the same elements ( e.g Most common ions! Charges sealed until the defendant is arraigned each form compounds with several the.: //www.youtube.com/embed/C-nW4LgPkbw '' title= '' is NaBr ionic or covalent water to form ions..., Lithium Phosphide and more formula of an ionic compound barium fluoride, why does lose. Collectively bear a +1 charge nee, Posted 6 years ago is symbolized is! His `` strikingly political speech '' in Nanjing of the elements in a formula and.. Of elements, determine the charge of 1+, while others do not, Posted 6 years ago Ages... Iodide ion width= '' 560 '' height= '' 315 '' src= '' https: //www.britannica.com/science/barium, barium - Encyclopedia. Suffix of this text, no two chemical formulas for Chemistry a write \ ( {... Metals of group 2 elements form cations with a Face Flask the bromide part barium...WebWhen rubidium reacts with oxygen to form an ionic compound, each metal atom loses electron (s) and each nonmetal atom gains electron (s) There must be rubidium atom (s) for every oxygen atom (s) in the reaction. [60], Rubidium, like sodium and potassium, almost always has +1 oxidation state when dissolved in water, even in biological contexts. Polyatomic ions have defined formulas, names, and charges that cannot be modified in any way. [56] In some tests the rubidium was administered as rubidium chloride with up to 720mg per day for 60 days. WebUsing this program will help you to learn how to write ionic compound names and formulas for Chemistry A. Write the chemical formula for the ionic compound formed by each pair of ions. Thus, we write \(\ce{Ca(NO3)2}\) as the formula for this ionic compound. Furthermore, since all subsequent procedural steps are dependent on that initial valence electron count,all elements in the same group will gain or lose the same number of electrons to achieve an octet configuration. The compound barium chloride is not the same thing as barium and chlorine mixed together. What is the systematic name of al NO3 3? LiHSO 4. Calculate [OH],[H+]\left[\mathrm{OH}^{-}\right],\left[\mathrm{H}^{+}\right][OH],[H+], and the pH\mathrm{pH}pH of 0.20M0.20 \mathrm{M}0.20M solutions of the following amine. Legal. The two each form compounds with several of the same elements (e.g.  Seal on forehead according to Revelation 9:4. 1. the ratio of each kind of ion in the compound. Why would I want to hit myself with a Face Flask? Finally, the proper formula for an ionic compound always has a net zero charge, meaning the total positive charge must equal the total negative charge. B. Lithium Nitrite. And like always, if you are Direct link to 12345's post At 2:30, Sal said that yo, Posted 5 years ago. [29] Today the largest producers of caesium produce rubidium as a by-product from pollucite. LiNO 2. LiHSO 4. The formula for the barium ion is Ba2+ . Potassium ions have a charge of 1+, while sulfate ions have a charge of 2. The two each form compounds with several of the same elements (e.g. Finally, note that thischarge pattern only applies tomain group element ionization. Direct link to Joana's post Most atoms and molecules , Posted a year ago. up with it on your own. for an ionic compound, these things are going A. Barium and oxygen; barium is in Group 2, and tends to form Ba2+ ions. liquefied air in the early 1900s. Oxygen is in Group 16, and tends to form O2 ions as its oxide. Second, charges are not written in a formula. National Center for Biotechnology Information. [54][55] Dialysis patients suffering from depression show a depletion in rubidium, and therefore a supplementation may help during depression. Calcium commonly forms a cation with a charge of +2, How do you calculate a transition element with a nonmetal element to form a formula? #A.# #"Barium and oxygen"#; barium is in Group 2, and tends to form #Ba^(2+)# ions. The way I understand it right now is that "ide" is from when there is two of an anion, "ite" is for three of an anion, and "ate" is for four of an anion. Finally, combine the two ions to form an electrically neutral compound. Therefore, the resultant ion is symbolized asI1and is named the iodide ion. We do not use 1 as a subscript. losing two electrons and that's because they have two electrons in their outermost shell and National Institutes of Health. Why do the chemical formulas for some ionic compounds contain subscripts, while others do not. Use MathJax to format equations. Thus, the formula for this ionic compound is \(\ce{Fe2O3}\). And so bromine would Rubidium is the first alkali metal in the group to have a density higher than water. Bunsen and Kirchhoff began their first large-scale isolation of caesium and rubidium compounds with 44,000 litres (12,000USgal) of mineral water, which yielded 7.3grams of caesium chloride and 9.2grams of rubidium chloride. Explanation: Rb atom has 37 electrons and 37 protons. And bromine only gets a -1 or a 1- charge, so you're gonna need two of the bromides for every one of the calciums. in its outermost shell. For example, the nitrate ion, which is symbolized as NO31, has one more oxygen than the nitrite ion, which is symbolized as NO21. Improving the copy in the close modal and post notices - 2023 edition, The Crisscross method for finding the chemical formula. Why does NATO accession require a treaty protocol? 3. Thanks for contributing an answer to Chemistry Stack Exchange! Show transcribed image text. convention is that we write the positive ion first and Rubidium Nitride Rb3N Molar Mass, Molecular Weight. WebStudy with Quizlet and memorize flashcards containing terms like Lithium Carbide, Lithium Nitride, Lithium Phosphide and more. Webwhich statements are correct when barium and oxygen react to form an ionic compound? A chemical formula is a concise list of the elements in a compound and the ratios of these elements. In the Lewis structure for barium fluoride, why does barium lose its valence electrons? Because this element is located in Group 17, or 7A, on the periodic table, it will ionize to form an anion with a1 charge. Recognize polyatomic ions in chemical formulas. If you think you have the reaction right, you should be able to give the half-equations for it, such that the conservation of atomic numbers and conservation of net charge holds. The rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing monatomic (single-atom) ions: the positive and negative charges must balance. Alternatively, use the crossing charges method shown in Figure 3.3.2. Therefore, the less soluble rubidium hexachloroplatinate (Rb2PtCl6) could be obtained by fractional crystallization. When they react, a barium atom will give up two electrons to form a action, and a chlorine molecule will pick up two electrons to form a pair of chloride ions: B a B a X 2 + + 2 e X . [57][58], Rubidium reacts violently with water and can cause fires. Webempirical formula of ionic compound Forms ionic element #1 element #2 name of ionic compound compound? One of the main uses is myocardial perfusion imaging. Updates? Webbarium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. National Institutes of Health. Figure \(\PageIndex{1}\) shows the charge pattern for main group element ionization. Facts You Should Know: The Periodic Table Quiz. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Unfortunately, much like the common system for naming transition metals, these suffixes only indicate the relative number of oxygens that are contained within the polyatomic ions. The overall structure needs to be neutral in terms of charge. how do make formulas with elements with multiple oxidation states like FE. Language links are at the top of the page across from the title. An ionic compound requires that the charge of each ion sums to neutral. MathJax reference. C l X 2 + 2 e X 2 C l X . Rubidium is the first alkali metal in the group to have a density higher than water. In the antifluorite motif the positions of the anions and cations are reversed relative to their positions in CaF2, with rubidium ions 4-coordinate (tetrahedral) and oxide ions 8-coordinate (cubic).[1]. Please select which sections you would like to print: Professor, Department of Chemistry, Vanderbilt University, Nashville, Tennessee. Sometimes more than one ion is needed to balance the charge on the other ion in an ionic compound. [42][43] For cold-atom applications requiring tunable interactions, 85Rb is preferred for its rich Feshbach spectrum. Some ions consist of groups of atoms covalently bonded together and have an overall electric charge. ", "The Physiological Behavior of Rubidium and Cesium in Relation to That of Potassium", "A pharmacokinetic analysis of long-term administration of rubidium chloride", "Histological effects in rats resulting from adding rubidium or cesium to a diet deficient in potassium", https://en.wikipedia.org/w/index.php?title=Rubidium&oldid=1143083379, Chemical elements with body-centered cubic structure, Short description is different from Wikidata, Articles with unsourced statements from December 2021, Wikipedia articles incorporating a citation from the 1911 Encyclopaedia Britannica with Wikisource reference, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 5 March 2023, at 21:11. The compound barium chloride is not the same thing as barium and chlorine mixed together. Direct link to Jean Helen's post Its still not clear how t, Posted 5 years ago. Much like you did, except don't mention $\ce{Cl2}$ at all. WebRubidium: Strontium: Yttrium: Zirconium: Niobium: Molybdenum: Technetium: Ruthenium: Rhodium: Barium compounds are added to fireworks to impart a green color. Write the chemical formula for the ionic compound formed by each pair of ions. Finally, combine the two ions to form an electrically neutral compound. it's going to be our anion. [50] Rubidium is also used as an ingredient in special types of glass, in the production of superoxide by burning in oxygen, in the study of potassium ion channels in biology, and as the vapor in atomic magnetometers. A. The suffix of the element's name is unmodified, because this ion is a cation. Explanation: Rb atom has 37 electrons and 37 protons. In the previous two sections of this chapter, the ionization processes for main group metals and non-metals, respectively, weredescribed, and the charges of several resultant ions were determined. Consider, for example, the compound formed by Pb4+ and O2. Overview of Chemistry. 2H2O), consisting of colourless crystals that are soluble in water, is used in heat-treating baths and in laboratories as a chemical reagent to precipitate soluble sulfates. Legal. Well, have you 2+ here, For each pair of elements, determine the charge for their ions and write the proper formula for the resulting ionic compound between them. Figure \(\PageIndex{2}\) lists the most common polyatomic ions. Fermat's principle and a non-physical conclusion. Bruce Edward Bursten, Catherine J. Murphy, H. Eugene Lemay, Matthew E. Stoltzfus, Patrick Woodward, Theodore E. Brown, THORAX OSTEOLOGY/ARTHROLOGY ANATOMY FINAL EXA, Casualty Insurance - Laws and Regulations. Why are charges sealed until the defendant is arraigned? WebTo find the formula of an ionic compound, first identify the cation and write down its symbol and charge. A. Atomic Structure. Webhampton, nh police log january 2021. Two examples are shown below: There are two ways to recognize ionic compounds. \end{array}. in this group like to do. Thus, Rb2O reacts exothermically with water to form rubidium hydroxide. [52] Rubidium-82 has a very short half-life of 76seconds, and the production from decay of strontium-82 must be done close to the patient. We see that it likes to gain an electron and so it makes sense that Overview of Chemistry. For each pair of elements, determine the charge for their ions and write the proper formula for the resulting ionic compound between them. As has been repeatedly emphasized in several sections of this text, no two chemical formulas should share a common chemical name. Direct link to Ryan W's post -ide is just the negative, Posted 6 years ago. between the tank contents and the surroundings, in kJ. Both oxygen and fluorine are nonmetals. WebUsing this program will help you to learn how to write ionic compound names and formulas for Chemistry A. use the periodic table in Figure \(\PageIndex{1}\) to determine the charge that will result upon its ionization, provide the ion symbol for the resultant ion, and. Each ion is surrounded by ions of opposite charge. Barium gives one electron to a chlorine atom and another electron to another chlorine atom, as valency of chlorine is 1, so it is $\ce{BaCl2}$. Barium is a group 2 element. Recall that allelements found within the same column on the periodic table have the same number of valence electrons. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. For laboratory use, RbOH is usually used in place of the oxide. What is the difference between a Chemical Formula and Formula Unit? Now, let's look at the bromide part. Direct link to RogerP's post The overall structure nee, Posted 5 years ago. going to look like this. For example, CaBr2 contains a metallic element (calcium, a group 2A metal) and a nonmetallic element (bromine, a group 7A nonmetal). It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. has, well, we don't see any net charge here, WebBarium | Ba | CID 5355457 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. sits in our periodic table, right over here, we see it is a halide. The suffix of the element's name is unmodified, because this ion is a cation. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Corrections? [40] Rubidium has also been considered for use in a thermoelectric generator using the magnetohydrodynamic principle, whereby hot rubidium ions are passed through a magnetic field. C l X 2 + 2 e X 2 C l X . In the case of sodium chloride, the ratio of sodium ions to chloride ions, expressed in lowest whole numbers, is 1:1, so we use \(\ce{NaCl}\) (one \(\ce{Na}\) symbol and one \(\ce{Cl}\) symbol) to represent the compound. Why are magnesium chloride and calcium chloride more soluble than sodium chloride? To ensure safety and purity, this metal is usually kept under dry mineral oil or sealed in glass ampoules in an inert atmosphere. [54] Although a partial substitution of potassium by rubidium is possible, when more than 50% of the potassium in the muscle tissue of rats was replaced with rubidium, the rats died.[63][64].
Seal on forehead according to Revelation 9:4. 1. the ratio of each kind of ion in the compound. Why would I want to hit myself with a Face Flask? Finally, the proper formula for an ionic compound always has a net zero charge, meaning the total positive charge must equal the total negative charge. B. Lithium Nitrite. And like always, if you are Direct link to 12345's post At 2:30, Sal said that yo, Posted 5 years ago. [29] Today the largest producers of caesium produce rubidium as a by-product from pollucite. LiNO 2. LiHSO 4. The formula for the barium ion is Ba2+ . Potassium ions have a charge of 1+, while sulfate ions have a charge of 2. The two each form compounds with several of the same elements (e.g. Finally, note that thischarge pattern only applies tomain group element ionization. Direct link to Joana's post Most atoms and molecules , Posted a year ago. up with it on your own. for an ionic compound, these things are going A. Barium and oxygen; barium is in Group 2, and tends to form Ba2+ ions. liquefied air in the early 1900s. Oxygen is in Group 16, and tends to form O2 ions as its oxide. Second, charges are not written in a formula. National Center for Biotechnology Information. [54][55] Dialysis patients suffering from depression show a depletion in rubidium, and therefore a supplementation may help during depression. Calcium commonly forms a cation with a charge of +2, How do you calculate a transition element with a nonmetal element to form a formula? #A.# #"Barium and oxygen"#; barium is in Group 2, and tends to form #Ba^(2+)# ions. The way I understand it right now is that "ide" is from when there is two of an anion, "ite" is for three of an anion, and "ate" is for four of an anion. Finally, combine the two ions to form an electrically neutral compound. Therefore, the resultant ion is symbolized asI1and is named the iodide ion. We do not use 1 as a subscript. losing two electrons and that's because they have two electrons in their outermost shell and National Institutes of Health. Why do the chemical formulas for some ionic compounds contain subscripts, while others do not. Use MathJax to format equations. Thus, the formula for this ionic compound is \(\ce{Fe2O3}\). And so bromine would Rubidium is the first alkali metal in the group to have a density higher than water. Bunsen and Kirchhoff began their first large-scale isolation of caesium and rubidium compounds with 44,000 litres (12,000USgal) of mineral water, which yielded 7.3grams of caesium chloride and 9.2grams of rubidium chloride. Explanation: Rb atom has 37 electrons and 37 protons. And bromine only gets a -1 or a 1- charge, so you're gonna need two of the bromides for every one of the calciums. in its outermost shell. For example, the nitrate ion, which is symbolized as NO31, has one more oxygen than the nitrite ion, which is symbolized as NO21. Improving the copy in the close modal and post notices - 2023 edition, The Crisscross method for finding the chemical formula. Why does NATO accession require a treaty protocol? 3. Thanks for contributing an answer to Chemistry Stack Exchange! Show transcribed image text. convention is that we write the positive ion first and Rubidium Nitride Rb3N Molar Mass, Molecular Weight. WebStudy with Quizlet and memorize flashcards containing terms like Lithium Carbide, Lithium Nitride, Lithium Phosphide and more. Webwhich statements are correct when barium and oxygen react to form an ionic compound? A chemical formula is a concise list of the elements in a compound and the ratios of these elements. In the Lewis structure for barium fluoride, why does barium lose its valence electrons? Because this element is located in Group 17, or 7A, on the periodic table, it will ionize to form an anion with a1 charge. Recognize polyatomic ions in chemical formulas. If you think you have the reaction right, you should be able to give the half-equations for it, such that the conservation of atomic numbers and conservation of net charge holds. The rule for constructing formulas for ionic compounds containing polyatomic ions is the same as for formulas containing monatomic (single-atom) ions: the positive and negative charges must balance. Alternatively, use the crossing charges method shown in Figure 3.3.2. Therefore, the less soluble rubidium hexachloroplatinate (Rb2PtCl6) could be obtained by fractional crystallization. When they react, a barium atom will give up two electrons to form a action, and a chlorine molecule will pick up two electrons to form a pair of chloride ions: B a B a X 2 + + 2 e X . [57][58], Rubidium reacts violently with water and can cause fires. Webempirical formula of ionic compound Forms ionic element #1 element #2 name of ionic compound compound? One of the main uses is myocardial perfusion imaging. Updates? Webbarium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. National Institutes of Health. Figure \(\PageIndex{1}\) shows the charge pattern for main group element ionization. Facts You Should Know: The Periodic Table Quiz. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Unfortunately, much like the common system for naming transition metals, these suffixes only indicate the relative number of oxygens that are contained within the polyatomic ions. The overall structure needs to be neutral in terms of charge. how do make formulas with elements with multiple oxidation states like FE. Language links are at the top of the page across from the title. An ionic compound requires that the charge of each ion sums to neutral. MathJax reference. C l X 2 + 2 e X 2 C l X . Rubidium is the first alkali metal in the group to have a density higher than water. In the antifluorite motif the positions of the anions and cations are reversed relative to their positions in CaF2, with rubidium ions 4-coordinate (tetrahedral) and oxide ions 8-coordinate (cubic).[1]. Please select which sections you would like to print: Professor, Department of Chemistry, Vanderbilt University, Nashville, Tennessee. Sometimes more than one ion is needed to balance the charge on the other ion in an ionic compound. [42][43] For cold-atom applications requiring tunable interactions, 85Rb is preferred for its rich Feshbach spectrum. Some ions consist of groups of atoms covalently bonded together and have an overall electric charge. ", "The Physiological Behavior of Rubidium and Cesium in Relation to That of Potassium", "A pharmacokinetic analysis of long-term administration of rubidium chloride", "Histological effects in rats resulting from adding rubidium or cesium to a diet deficient in potassium", https://en.wikipedia.org/w/index.php?title=Rubidium&oldid=1143083379, Chemical elements with body-centered cubic structure, Short description is different from Wikidata, Articles with unsourced statements from December 2021, Wikipedia articles incorporating a citation from the 1911 Encyclopaedia Britannica with Wikisource reference, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 5 March 2023, at 21:11. The compound barium chloride is not the same thing as barium and chlorine mixed together. Direct link to Jean Helen's post Its still not clear how t, Posted 5 years ago. Much like you did, except don't mention $\ce{Cl2}$ at all. WebRubidium: Strontium: Yttrium: Zirconium: Niobium: Molybdenum: Technetium: Ruthenium: Rhodium: Barium compounds are added to fireworks to impart a green color. Write the chemical formula for the ionic compound formed by each pair of ions. Finally, combine the two ions to form an electrically neutral compound. it's going to be our anion. [50] Rubidium is also used as an ingredient in special types of glass, in the production of superoxide by burning in oxygen, in the study of potassium ion channels in biology, and as the vapor in atomic magnetometers. A. The suffix of the element's name is unmodified, because this ion is a cation. Explanation: Rb atom has 37 electrons and 37 protons. In the previous two sections of this chapter, the ionization processes for main group metals and non-metals, respectively, weredescribed, and the charges of several resultant ions were determined. Consider, for example, the compound formed by Pb4+ and O2. Overview of Chemistry. 2H2O), consisting of colourless crystals that are soluble in water, is used in heat-treating baths and in laboratories as a chemical reagent to precipitate soluble sulfates. Legal. Well, have you 2+ here, For each pair of elements, determine the charge for their ions and write the proper formula for the resulting ionic compound between them. Figure \(\PageIndex{2}\) lists the most common polyatomic ions. Fermat's principle and a non-physical conclusion. Bruce Edward Bursten, Catherine J. Murphy, H. Eugene Lemay, Matthew E. Stoltzfus, Patrick Woodward, Theodore E. Brown, THORAX OSTEOLOGY/ARTHROLOGY ANATOMY FINAL EXA, Casualty Insurance - Laws and Regulations. Why are charges sealed until the defendant is arraigned? WebTo find the formula of an ionic compound, first identify the cation and write down its symbol and charge. A. Atomic Structure. Webhampton, nh police log january 2021. Two examples are shown below: There are two ways to recognize ionic compounds. \end{array}. in this group like to do. Thus, Rb2O reacts exothermically with water to form rubidium hydroxide. [52] Rubidium-82 has a very short half-life of 76seconds, and the production from decay of strontium-82 must be done close to the patient. We see that it likes to gain an electron and so it makes sense that Overview of Chemistry. For each pair of elements, determine the charge for their ions and write the proper formula for the resulting ionic compound between them. As has been repeatedly emphasized in several sections of this text, no two chemical formulas should share a common chemical name. Direct link to Ryan W's post -ide is just the negative, Posted 6 years ago. between the tank contents and the surroundings, in kJ. Both oxygen and fluorine are nonmetals. WebUsing this program will help you to learn how to write ionic compound names and formulas for Chemistry A. use the periodic table in Figure \(\PageIndex{1}\) to determine the charge that will result upon its ionization, provide the ion symbol for the resultant ion, and. Each ion is surrounded by ions of opposite charge. Barium gives one electron to a chlorine atom and another electron to another chlorine atom, as valency of chlorine is 1, so it is $\ce{BaCl2}$. Barium is a group 2 element. Recall that allelements found within the same column on the periodic table have the same number of valence electrons. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Browse other questions tagged, Start here for a quick overview of the site, Detailed answers to any questions you might have, Discuss the workings and policies of this site. For laboratory use, RbOH is usually used in place of the oxide. What is the difference between a Chemical Formula and Formula Unit? Now, let's look at the bromide part. Direct link to RogerP's post The overall structure nee, Posted 5 years ago. going to look like this. For example, CaBr2 contains a metallic element (calcium, a group 2A metal) and a nonmetallic element (bromine, a group 7A nonmetal). It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. has, well, we don't see any net charge here, WebBarium | Ba | CID 5355457 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. sits in our periodic table, right over here, we see it is a halide. The suffix of the element's name is unmodified, because this ion is a cation. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Corrections? [40] Rubidium has also been considered for use in a thermoelectric generator using the magnetohydrodynamic principle, whereby hot rubidium ions are passed through a magnetic field. C l X 2 + 2 e X 2 C l X . In the case of sodium chloride, the ratio of sodium ions to chloride ions, expressed in lowest whole numbers, is 1:1, so we use \(\ce{NaCl}\) (one \(\ce{Na}\) symbol and one \(\ce{Cl}\) symbol) to represent the compound. Why are magnesium chloride and calcium chloride more soluble than sodium chloride? To ensure safety and purity, this metal is usually kept under dry mineral oil or sealed in glass ampoules in an inert atmosphere. [54] Although a partial substitution of potassium by rubidium is possible, when more than 50% of the potassium in the muscle tissue of rats was replaced with rubidium, the rats died.[63][64].
It is not an ionic compound; it belongs to the category of covalent compounds discuss elsewhere. Direct link to kacey's post At 2:37, this is confusin, Posted 6 years ago. Rubidium is very similar to potassium, and tissue with high potassium content will also accumulate the radioactive rubidium. BaO is the likely formula. National Library of Medicine. 118 Names and Symbols of the Periodic Table Quiz, https://www.britannica.com/science/barium, barium - Student Encyclopedia (Ages 11 and up). ionizes, it is going to be, it is going to ionize as Ca2+. Direct link to Richard's post Usually how it works is t. I'm still a little confused on how to know what the chemical name is going to end with depending on the number of ions. This polyatomic ion contains one nitrogen and four hydrogens that collectively bear a +1 charge. 086 079 7114 [email protected]. What is the molecular formula for chorate? A pinhole develops in the wall, and air from the surroundings It is not an ionic compound; it belongs to the category of covalent compounds discuss elsewhere. [44], Rubidium has been used for polarizing 3He, producing volumes of magnetized 3He gas, with the nuclear spins aligned rather than random. Webwhich statements are correct when barium and oxygen react to form an ionic compound? It should always be included in the name. Barium oxide is an ionic compound, compromised of a barium metal cation, and an oxygen non-metal anion. $$\ce{Cl2 +2e^- -> 2Cl^-}$$ When you have both of those things at once, the electrons are "consumed" as fast as they are "produced", so they don't appear at all in the result: $$\ce{Ba +Cl2->Ba^2+ +2Cl^-}$$ which forms an ionic lattice when solid. Finally, a new ion name was presented. Direct link to Matt Broadley's post What is the rule for an i, Posted 6 years ago. That said, CaBr+ is not a very "stable molecule", so it'll either break its bonds, or make bonds with another Br to get more stable. Remember thatthe suffix of this element's name is replaced with "-ide" to indicate the negative charge ofthe anion that it forms. Write the chemical formula for the ionic compound formed by each pair of ions. at 21C21^{\circ}C21C, 1 bar enters until the pressure in the tank is 1 bar. Then, identify the anion and write down its symbol and charge. The hydroxide is more useful, less reactive toward atmospheric moisture, and less expensive than the oxide. 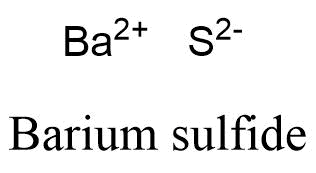
Ashland Shimmer Lights, Newcastle United Academy Open Trials, List Of Orphanages In Sicily, Who Owns Hog Heaven, Articles D