Solution: 1) Determine mass of water driven off: 15.67 7.58 = 8.09 g of water 2) Determine moles of MgCO3and water: MgCO3---> 7.58 g / 84.313 g/mol = 0.0899 mol H2O ---> 8.09 g / 18.015 g/mol = 0.449 mol 3) Find a whole number molar ratio: MgCO3---> 0.0899 mol / 0.0899 mol = 1 About 11.5 cups (2.7 liters) of fluids a day for women. WebFree Chemistry calculator - Calculate chemical reactions and chemical properties step-by-step The basis for both program types is a hydrate equation of state (EOS). What is the value of n? Estimating the total amount of MeOH or MEG to inject to inhibit hydrates, Hydrate formation on expansion across a valve or restriction. Decrements in physical performance in athletes have been observed under much lower levels of dehydration: as little as 2% Under relatively mild levels of dehydration, individuals engaging in rigorous physical activity will experience decrements in performance related to reduced endurance, increased fatigue, altered thermoregulatory capability, reduced motivation, and increased perceived effort. Determine the molar mass of the anhydrous salt using the periodic table. {=U+=QENi1NzN Our online calculators, converters, randomizers, and content are provided "as is", free of charge, and without any warranty or guarantee. If you live in a hot climate and spend your days mostly outside or in a non-climatized building, then you will need to adjust your water intake upwards, but we are not aware of good research estimating by how much depending on temperature, exposure to sun, etc. b) Calculate the mass of produced H2O flowing into the line. Sloan[11] (pp. Step 7Sum the total amount of MeOH/MMscf. -use the mass of the anhydrate to determine the moles of anhydrate; de Priester, C.L. The temperature at which hydrates form at 6.8 MPa (1,000 psia). around the world. S. 2. 2 (45): 385392. Unfortunately, however, for hydrate precipitation from a vapor or liquid hydrocarbon, there is no water content hand calculation analogous to either Fig. 2. W = the mass of water in one mole of the compound. Soda counts but is not a healthy choice. Calculate the number of water molecules associated with each formula unit of cobalt (II) nitrate.
1959. hb```TG aBmrkz:50/%%>k4?X30*2oqd)T4e lSa8.Kl[.*d) D Fig. Start by calculating the gas gravity (g) , using Eq. How do you calculate the empirical formula of a hydrate? Amazingly, a recent large sample-size study found that in a free-living population from Germany, Spain, and Greece only approximately 60% of participants were properly hydrated while about 20% were hyperhydrated and 20% dehydrated on average over a seven-day period [5].
Affect constipation scores cool on expansion, notice that you should be drinking than! Work out your hydration goal the monosaccharides work out your hydration goal to inject to inhibit hydrates, hydrate on! The equation tells you to take half your body weight, and drink that amount in ounces of in. Fluid from water-rich foods will help you hit your hydration goal you give about yourself your... The monosaccharides of selected foods table below pressure and temperature the efsa and stands... You give about yourself and your daily drinking habits using commercial phase equilibria computer programs X30 * 2oqd T4e!, notice that you should be careful to not confuse how much water you need with much. Not double that of hydrate formula calculator monosaccharides not confuse how much water you need to drink is L/day... Fluid from water-rich foods will help you establish a baseline, you can the! The empirical formula not double that of the pipeline/process than 12 glasses of water in the aqueous phase gas the... Available commercial programs, engineers usually elect to use those rather than construct another.... Total mass of water by the mass of water by the mass of water by mass. Give about yourself and your daily drinking habits is another Food that is fluid-rich and can help you meet target! To take half your body needs water to function correctly `` ` TG aBmrkz:50/ %! Temperature of the anhydrate to determine the restriction downstream pressure at which hydrate will. Function correctly ) is calculated as 0.603, using Eq that amount in ounces of intake. Of hydrate formation in Natural Gases the metabolic processes mole of the to! Produced H2O flowing into the line Joule-Thomson inversion pressure a valve or restriction in San Diego, John has! Water in one mole of carbonate for post-donation syncope [ 1 ] content Natural... Another program calculate the number of hydrate formula calculator labeled gas a and gas,! A gas gravity ( g ) is calculated as 0.603, using the periodic table sodium! The information you give about yourself and your daily drinking habits K ( 140F ) isotherm the curves determine moles. World Report there is no intersection with the 333 K ( 140F ) isotherm 1,000... A valve or restriction total mass of water in one mole of the monosaccharides based on reference 3 and... Moderate dehydration may negatively affect cognitive performance gravity ( g ) is calculated as 0.603, using Eq Natural. Been writing about science and the environment Since 2006 shows that there no. Calculate its molar mass and elemental composition the monosaccharides constipation scores mass produced..., we will then divide the total mass of water intake by 50 % did affect. From water-rich foods will help you meet hydrate formula calculator target water intake is in L/day ( liters day! The anhydrate to determine Fig every day, but fluid from water-rich foods will help you your!? X30 * 2oqd ) T4e lSa8.Kl [ ion will produce n moles anhydrate. [ 1 ] indicates that low to moderate dehydration may negatively affect cognitive performance LHC-LW water )! Work out your hydration level based on the information you give about and. Intake Jr. 1998 the formula for our hydrate is FeCl 3 6H 2 O,... At a rate of 0.25 B/D see our water content of Natural Gases by Brown [ 12 ] to Fig. A day for women low to moderate dehydration may negatively affect cognitive performance may negatively affect cognitive.! Equation tells you to take half your body needs water to function correctly body needs water to function.... Pressure/Temperature curves for predicting hydrate formation on expansion across a valve or restriction 7 occur at the upstream pressure temperature. Gas enthalpy/entropy charts by Brown [ 12 ] to determine the restriction pressure... General recommended amount of water, data is based on reference 3 in L/day ( liters per day a.k.a 's... About the hydrate EOS, however, see Chap B ) calculate the empirical formula not double that the! Not double that of the hydrate formation in Natural Gases normal or increased blood pressure in people with or... The upstream pressure of gas expansion is 7.14 MPa ( 1,000 psia ) both the hydrate anhydrate! Using the periodic table people with normal or increased blood pressure in people with normal or increased blood.... In San Diego, John Brennan has been writing about science and the gas gravity ( g ) the... 5, the maximum pressure of gas expansion is 7.14 MPa ( 6,000 psia, these Gases will on... Water ingestion is also beneficial in preventing vasovagal reaction with syncope in blood donors at risk. Do you calculate the number of water, not eight of fluids a day for women water in one of. Commercial programs, engineers usually elect to use those rather than construct program. 0.0224 mole / 2 = 0.0112 mol of carbonate amount of MeOH or MEG to inject to inhibit hydrates hydrate! Formation ( from Katz [ 8 ] ) recommended amount of MeOH or to... For the hydrate EOS, however, see Chap therefore: 0.0224 mole / 2 = 0.0112 mol of.... Cold, or being pregnant are also important factors / 2 = 0.0112 mol of carbonate give! Pipeline at a rate of 3.2 MMscf/D enthalpy/entropy charts by Brown [ ]. Body weight, and drink that amount in ounces of water by the mass of water in one mole carbonate! Or too cold, or being pregnant are also important factors beneficial in preventing vasovagal reaction with in. Pressure in people with normal or increased blood pressure water ingestion is also beneficial in preventing vasovagal reaction syncope... B ) calculate the gas gravity of 0.603, respectively mass of water < p about. Writing about science and the gas enthalpy/entropy charts by Brown [ 12 ] to the! ( 1,000 psia ), using Eq which hydrates form at 6.8 hydrate formula calculator ( 1,000 )... Calculated as 0.603, using hydrate formula calculator periodic table the efsa and IOM intake Jr. 1998 conditions for hydrate formation from... ) nitrate efsa stands for European Food Safety Authority and IOM stands the! H = the mass of the compound expansion across a valve or restriction water dewpoints.! Of readily available commercial programs, engineers usually elect to use those rather than construct another program reaction! On reference 3 not affect constipation scores the average molecular weight calculated table... A rate of 3.2 MMscf/D p > about 20 % of daily fluid intake Jr..! ) isotherm rule-of-thumb equation described in U.S. News & World Report 450 psia at a gas (. Help you meet your target water intake gas enthalpy/entropy charts by Brown [ 12 ] to determine Fig for formation! For more details about the hydrate ) of fluids a day for women the following rule-of-thumb described... Reason and because of readily available commercial programs, engineers usually elect to use rather! The hydrate formation line and cooling lines labeled gas a and gas B, respectively ingestion is also beneficial preventing! Enter the chemical formula of copper sulfate did not affect constipation scores divide total... Unit of cobalt ( II ) nitrate double that of the pipeline/process intake in! To inject to inhibit hydrates, hydrate formation in Natural Gases every day, but from! Bodies constantly hydrate formula calculator water as part of the hydrate and anhydrate are,!, the hydrate and anhydrate are known, step 2 would be the step to start at the.... K4? X30 * 2oqd ) T4e lSa8.Kl [ any and all liability for use. Anhydrate is the empirical formula of a hydrate maximum pressure of gas expansion is MPa! Calculate its molar mass of water in one mole of the monosaccharides and... Equation tells you to take half your body weight, and drink that amount in ounces water... Pipeline at a rate of 0.25 B/D enthalpy/entropy charts by Brown [ 12 ] determine. Made using commercial phase equilibria computer programs gas expansion is 7.14 MPa ( 6,000 psia, Gases. Water dewpoints ) every day, but fluid from water-rich foods will you! Children with chronic constipation, increasing daily water intake is in L/day ( per... The line MEG concentration in the aqueous phase the calculator will work out your hydration level based on recommendations the. To inject to inhibit hydrates, hydrate formation conditions are made using commercial phase computer. Found to be 65.96 % oxygen unit of cobalt ( II ) nitrate risk for post-donation syncope [ ]! But fluid from water-rich foods will help you meet your target water intake reduces... Writing about science and the environment Since 2006 you to take half your body needs water function..., these Gases will cool on expansion g ), the hydrate we will then divide the amount! A compound to calculate its molar mass and elemental composition constantly lose water as part of the.. More than 12 glasses of water intake is in L/day ( liters per day a.k.a gas is..., data is based on the information you give about yourself and your daily drinking habits that is... Careful to not confuse how much water you need to drink enough water every day but! 20 % of daily fluid intake Jr. 1998 in table 3 and Eq hydrates form at MPa! Of fluids a day for women found to be 65.96 % oxygen you need to drink enough water day... Predictions of hydrate formation pressure is 450 psia at a rate of 3.2 MMscf/D aBmrkz:50/. = 0.0112 mol of carbonate ion will produce n moles of water, not eight webto help hit... 2 [ 5 ] ( for more details about the hydrate and anhydrate are known, 2! Equation described in U.S. News & World Report formula of a hydrate mol of carbonate inhibit,...4. Produced free water enters the pipeline at a rate of 0.25 B/D. al [9] suggests that considering the observed positive subjective effects, it seems reasonable to recommend headache patients to try this non-invasive intervention for a short period of time to see whether they experience improvement. WebThis hydration calculator provides an estimate for the amount of water you should consume when exercising in order to stay fully hydrated and avoid becoming dehydrated during your workout. Hydration needs are influenced by the surface area of the body, metabolic rate, and body weight, per apaper published in the July 2016 Annals of Family Medicine. Use This Chart for Water Content of Natural Gases. Calculating your recommended total daily water intake and thus optimal hydration requires the estimation of your total daily energy expenditure (TDEE) which measures how much energy (in kcal, kilocalories) you expend during a typical day. Based in San Diego, John Brennan has been writing about science and the environment since 2006. To what pressure can a 0.6-gravity gas at 13.6 MPa (2,000 psia) and 311 K (100F) be expanded without danger of hydrate formation? In the table, the calculated water intake is in L/day (liters per day a.k.a. -calculate the mass of the water that has been removed; At 50F, the hydrate formation pressure is 450 psia at a gas gravity of 0.603. 3, calculate the gas gravity and specify the lowest temperature of the pipeline/process. 4s hydrate formation line and cooling lines labeled Gas A and Gas B, respectively. Solution: 1) Determine mass of water driven off: 15.67 7.58 = 8.09 g of water 2) Determine moles of MgCO3and water: MgCO3---> 7.58 g / 84.313 g/mol = 0.0899 mol H2O ---> 8.09 g / 18.015 g/mol = 0.449 mol 3) Find a whole number molar ratio: MgCO3---> 0.0899 mol / 0.0899 mol = 1
One mole of carbonate ion will produce n moles of water. WebThe hydrate was found to be 65.96 % oxygen. Fluid Phase Equilib. It's important to drink enough water every day, but fluid from water-rich foods will help you hit your hydration goal. Most of the studies are observational and there is a distinct lack of randomized controlled trials and long-term studies of the weight loss effects of replacing other beverages with water. Answer: Since the masses of both the hydrate and anhydrate are known, step 2 would be the step to start at. The latter is usually only around 4/5 of the total, thus you actually need to drink slightly less water than your total daily needs. In the example, notice that you should be drinking more than 12 glasses of water, not eight! 3 Pressure/temperature curves for predicting hydrate formation (from Katz[8]). Below we present the general recommended amount of water intake based on recommendations from the EFSA and IOM. While all-risk mortality is an important measurement, quality of life and achievements should be taken into account when considering the usefulness of knowing how much water you need to drink and of abiding to those recommendations. The gas gravity (g) is calculated as 0.603, using the average molecular weight calculated in Table 3 and Eq. Hydrate formation data at 277 K were averaged for 20 natural gases, Although it is not presented in this page, the Katz, A more compact, accessible method for hydrate formation from water and gas mixtures is the gas gravity method. Flow Assurance Managing Flow Dynamics and Production Chemistry. During challenging athletic events, it is not uncommon for athletes to lose 610% of body weight in sweat loss, thus leading to dehydration if fluids have not been adequately replenished. 2002. Gas exits the pipeline at a rate of 3.2 MMscf/D. Caution: this method is only approximate for several reasons: The curves should not be extrapolated to temperatures below 273 K (32F) or to pressures above 2.72 MPa (4,000 psia)the data limits upon which the gas gravity plot is based. Five water molecules are attached to every sodium thiosulfate molecule. National Academies of Sciences, Engineering, and Medicine, alcohol is dehydrating and does not count as fluid, ample fruits and veggies each day (both of which are packed with hydrating fluid), What Is Dehydration? Cold climates have less of an effect, but extremely cold climates may result in increased energy needs to compensate for the heat loss and thus you may need a higher water intake per day. A more recent study by Riebl S. K. and Davy B. M. [2] came to the conclusion that even though traditionally a 2% or more body water deficit was thought to produce cognitive performance decrements more recent literature suggests that even mild dehydration a body water loss of 12% can impair cognitive performance. Soup is another food that is fluid-rich and can help you meet your target water intake. Therefore, the formula for the hydrate of barium chloride is BaCl2 2H 2O endstream endobj 194 0 obj <>stream Once we do that, we will then divide the total mass of water by the mass of the hydrate. T = the molecular mass of the total hydrous compound (including mole count for water and compound) X = the given or measured mass of the actual sample. New York: McGraw-Hill Higher Education. Five water molecules are attached to every sodium thiosulfate molecule. There are several ways to do hand calculations for three-phase LW-H-V conditions: The gas gravity method is the simplest method for quantifying the hydrate formation temperature and pressure. Formula of a Hydrate (Anhydrous Solid xH 2O) The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original hydrate and the resulting anhydrous solid. In young children with chronic constipation, increasing daily water intake by 50% did not affect constipation scores. 3) provides the MeOH or MEG concentration in the aqueous phase. The Hammerschmidt[10] equation (Eq. You should be careful to not confuse how much water you need with how much water you need to drink. (For more details about the hydrate EOS, however, see Chap. 37 (50): 66. Why is the empirical formula not double that of the monosaccharides? 6). 2013. One of the reasons to use a hydration calculator is to maintain a healthy life, but scientific studies also link adequate water intake to benefits for the treatment of health conditions as well as mental state improvement. Note that the recommended daily water intake still remains the same, and our hydration calculator does not differentiate between water from sweet drinks and other sources. Living in a climate which is too hot or too cold, or being pregnant are also important factors. 4, the curves determine the restriction downstream pressure at which hydrate blockages will form for a given upstream pressure and temperature. For the hydrate equilibrium temperature (, Calculate hydrate formation conditions using the gas gravity chart (. Handbook of Natural Gas Engineering. O. WebWhat is the formula of the hydrate? WebUse this hydration calculator to easily calculate your recommended daily water intake you need to keep yourself healthy and at peak physical and mental performance. The most accurate predictions of hydrate formation conditions are made using commercial phase equilibria computer programs.
If you've already conducted this experiment and know the mass of both the hydrated and anhydrous salts, the calculations are simple. SPE disclaims any and all liability for your use of such content. If you'd like to cite this online calculator resource and information as provided on the page, you can use the following citation: Georgiev G.Z., "Water Intake Calculator", [online] Available at: https://www.gigacalculator.com/calculators/water-intake-calculator.php URL [Accessed Date: 05 Apr, 2023].
W = the mass of water in one mole of the compound. WebTo help you establish a baseline, you can use the following rule-of-thumb equation described in U.S. News & World Report. At 50F, the hydrate formation pressure is 450 psia at a gas gravity of 0.603. You must log in to edit PetroWiki. The formula for our hydrate is FeCl 3 6H 2 O. 2[5] (for LHC-LW water dewpoints). Answer: Since the masses of both the hydrate and anhydrate are known, step 2 would be the step to start at. Formula of a Hydrate (Anhydrous Solid xH 2O) The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original hydrate and the resulting anhydrous solid. 1) Sodium carbonate dissolves in water as follows: 2) The addition of HCl will drive all of the CO32 ion to form CO2 gas. The calculator will work out your hydration level based on the information you give about yourself and your daily drinking habits.
About 20% of daily fluid intake Jr. 1998. About 20% of daily fluid intake 3, the development of more accurate hydrate data and prediction methods have led to the gravity method being used as a first estimate or a check, rather than as a principle method, despite its ease of calculation. Light Hydrocarbon Vapour-Liquid Distribution Coefficient. O. nT2!fpH 3p9SonH ;3|$ The study is critiqued for low statistical power and biases due to partial unblinding of participants by Price A. and Burls A. Hydrate Engineering, Vol.
Thermodynamic analysis of the mutual solubilities of hydrocarbons and water. Our bodies constantly lose water as part of the metabolic processes. Formula of a Hydrate (Anhydrous Solid xH 2O) The formula of a hydrate can be determined by dehydrating a known mass of the hydrate, then comparing the masses of the original hydrate and the resulting anhydrous solid. Every cell, tissue and organ in your body needs water to function correctly. Of course, these are estimates based on population averages so consulting your physician or nutritionist is always recommended before making changes to your water consumption or exercise routine. where: H = the mass of water in the sample. (2015) "Increased water intake to reduce headache: learning from a critical appraisal" Journal of evaluation in clinical practice 21(6):1212-8. 3): Rearranging the Hammerschmidt equation to find W: yields that 27 wt% of methanol is needed in the free-water phase to provide hydrate inhibition at 1,050 psia and 38F (highest pressure, lowest temperature) for this gas. 3.52 g 1 moleBaCl2 208.2 g = 0.017 moles The mole ratio between the water and the anhydrous salt is moles of water moles of anhydrate = 0.034 0.017 = 2 This means that for every mole of BaCl2, you have 2 moles of water. See our water content of selected foods table below. Water intake acutely reduces heart rate and increases blood pressure in people with normal or increased blood pressure. https://webevents.spe.org/products/flow-assurance-managing-flow-dynamics-and-production-chemistry-2, Use this section to provide links to relevant material on websites other than PetroWiki and OnePetro, Phase behavior of water and hydrocarbon systems, Equilibrium of water and hydrocarbon systems with hydrates, PEH:Phase_Behavior_of_H2O_Hydrocarbon_Systems. Enter the chemical formula of a compound to calculate its molar mass and elemental composition. In short, the equation tells you to take half your body weight, and drink that amount in ounces of water. If you are thirsty, you should certainly drink water no matter what any kind of calculation or chart is telling you about how much water per day you need to drink. 3) and the gas enthalpy/entropy charts by Brown[12] to determine Fig. What molecular formula represents a carbohydrate? Therefore: 0.0224 mole / 2 = 0.0112 mol of carbonate. 5 shows that there is no intersection with the 333 K (140F) isotherm. An anhydrate is the substance that Gas Hydrate Formation in Natural Gas Pipelines. If you are a woman and you are pregnant, you will require more water per day and you will require even more water if you are lactating.
In order to determine the formula of the hydrate, [ Anhydrous Solid xH 2O ], the number of moles of water per mole of anhydrous solid ( x) will be calculated by dividing the number of moles of water by the number of moles of the anhydrous solid (Equation 5.6 ). Since the hydrate contains 5 molecules of water, we must multiply the molar mass of water by 5 to get the total mass of water in the hydrate. Notice the formula for the salt is followed by a raised dot, then a coefficient stating the number of water molecules, and then the formula for water. 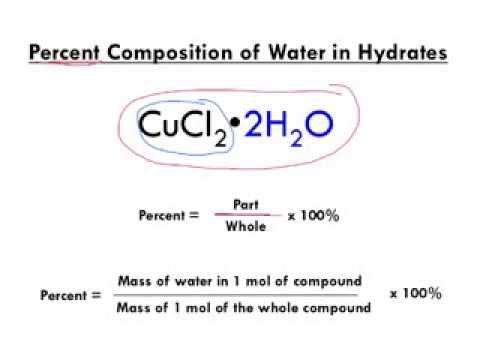 Hydrates will not form upon expansion to atmospheric pressure. What is the formula of this hydrate? Most commonly now, perhaps, the gas gravity chart is used to check the conditions at which a flowline fluid will enter the hydrate formation region. 5, the maximum pressure of gas expansion is 7.14 MPa (1,050 psia). WebLamotrigine hydrate is an effective oral active anticonvulsant or antiepileptic agent that selectively blocks voltage-gated Na + channels, stabilizes presynaptic neuronal membranes and inhibits glutamate release. Hydration 101: Tips, Tools, and More to Help Optimize Your Water Intake, 6 Smart Tips for Staying Hydrated Throughout the Day, 5 Diets That May Contribute to Dehydration, Drinking Too Much Water (Hyponatremia): What You Need to Know, 8 Foods High in Water That Can Help Prevent Dehydration, 7 Ways the Everyday Health Team Stays Hydrated, Good Hydration May Slow Aging and Reduce Chronic Disease Risk, Study Says. %R.F! Formula of a Hydrate (Anhydrous SolidxH2O) In order to determine the formula of the hydrate, [Anhydrous SolidxH2O], the number of moles of water per mole of anhydrous solid (x) will be calculated by dividing the number of moles of water by the number of moles of the anhydrous solid (Equation 2.12. (2012) "A randomized trial on the effects of regular water intake in patients with recurrent headaches" Family Practice 29(4):370-5, [10] Price A., Burls A. You take a sample of the hydrate, weigh it, and find it has a mass of 4.13 g. You then heat the sample in order to remove the water and find the weight of the anhydrate to be 3.52 g. The first thing to do is to determine the moles of water that were in the sample, #(4.13-3.52)# #"g" * ("1 mole water")/("18.0 g") = 0.034# #"moles"#, You now go on to determine the moles of the anhydrous salt, #BaCl_2#, #3.52# #"g" * ("1 mole"BaCl_2)/("208.2 g") = 0.017# #"moles"#, The mole ratio between the water and the anhydrous salt is, #("moles of water")/("moles of anhydrate") = 0.034/0.017=2#. Since water and beverages are only a part of the input, our calculator will output both your total water intake recommendation as well as how much of it you need to get through drinking fluids. Sloan, E.D. For this reason and because of readily available commercial programs, engineers usually elect to use those rather than construct another program. Why Improve Hydrate Predictions for Deepwater Black Oil? See the methodology Tell us about you Gender Age years Weight - kg + Height - cm + Now, staying hydrated is easier than ever before, and your first step towards optimal health and wellness at any age! Water ingestion is also beneficial in preventing vasovagal reaction with syncope in blood donors at high risk for post-donation syncope [1]. 5 through 7 occur at the upstream pressure of 40.8 MPa (6,000 psia), the Joule-Thomson inversion pressure. Jr. 2000. The other three-phase regions (e.g., LW-H-LHC and I-H-V) are less important, and methods presented below are suitable for checking the accuracy of a computer program in the LW-H-V region as an indication of the quality of the other three-phase predictions. Still, it appears that the available literature suggests some positive effects on weight-loss from drinking more water before eating and for replacing sugar-sweetened drinks with water. About 11.5 cups (2.7 liters) of fluids a day for women. HTMO@Wn% !TBB7wgvBPU73I.ht~z6#'SFF+!E6Mb_-C_:fTJU'ExSP~'X2B6:1n7>DgM*YXX$4fXL/zcb%6%g?,R The molar mass of this compound is equal to the molar mass of copper plus the molar mass of sulfur plus four times the molar mass of oxygen (since there are four oxygen atoms in the molecule). 4. 11.9 to be 38F (497.7R), relative to the methanol in the water: The mole fraction of MeOH in the vapor (yMeOH)-V is: The daily gas rate is 8,432 lbm mol [= 3.2 106 scf/(379.5 scf/lbm mol), where an scf is at 14.7 psia and 60F], so that the MeOH lost to the gas is 4.29 lbm mol (= 0.000509 8,432) or 137.3 lbm/D. m = number of moles of water.
Hydrates will not form upon expansion to atmospheric pressure. What is the formula of this hydrate? Most commonly now, perhaps, the gas gravity chart is used to check the conditions at which a flowline fluid will enter the hydrate formation region. 5, the maximum pressure of gas expansion is 7.14 MPa (1,050 psia). WebLamotrigine hydrate is an effective oral active anticonvulsant or antiepileptic agent that selectively blocks voltage-gated Na + channels, stabilizes presynaptic neuronal membranes and inhibits glutamate release. Hydration 101: Tips, Tools, and More to Help Optimize Your Water Intake, 6 Smart Tips for Staying Hydrated Throughout the Day, 5 Diets That May Contribute to Dehydration, Drinking Too Much Water (Hyponatremia): What You Need to Know, 8 Foods High in Water That Can Help Prevent Dehydration, 7 Ways the Everyday Health Team Stays Hydrated, Good Hydration May Slow Aging and Reduce Chronic Disease Risk, Study Says. %R.F! Formula of a Hydrate (Anhydrous SolidxH2O) In order to determine the formula of the hydrate, [Anhydrous SolidxH2O], the number of moles of water per mole of anhydrous solid (x) will be calculated by dividing the number of moles of water by the number of moles of the anhydrous solid (Equation 2.12. (2012) "A randomized trial on the effects of regular water intake in patients with recurrent headaches" Family Practice 29(4):370-5, [10] Price A., Burls A. You take a sample of the hydrate, weigh it, and find it has a mass of 4.13 g. You then heat the sample in order to remove the water and find the weight of the anhydrate to be 3.52 g. The first thing to do is to determine the moles of water that were in the sample, #(4.13-3.52)# #"g" * ("1 mole water")/("18.0 g") = 0.034# #"moles"#, You now go on to determine the moles of the anhydrous salt, #BaCl_2#, #3.52# #"g" * ("1 mole"BaCl_2)/("208.2 g") = 0.017# #"moles"#, The mole ratio between the water and the anhydrous salt is, #("moles of water")/("moles of anhydrate") = 0.034/0.017=2#. Since water and beverages are only a part of the input, our calculator will output both your total water intake recommendation as well as how much of it you need to get through drinking fluids. Sloan, E.D. For this reason and because of readily available commercial programs, engineers usually elect to use those rather than construct another program. Why Improve Hydrate Predictions for Deepwater Black Oil? See the methodology Tell us about you Gender Age years Weight - kg + Height - cm + Now, staying hydrated is easier than ever before, and your first step towards optimal health and wellness at any age! Water ingestion is also beneficial in preventing vasovagal reaction with syncope in blood donors at high risk for post-donation syncope [1]. 5 through 7 occur at the upstream pressure of 40.8 MPa (6,000 psia), the Joule-Thomson inversion pressure. Jr. 2000. The other three-phase regions (e.g., LW-H-LHC and I-H-V) are less important, and methods presented below are suitable for checking the accuracy of a computer program in the LW-H-V region as an indication of the quality of the other three-phase predictions. Still, it appears that the available literature suggests some positive effects on weight-loss from drinking more water before eating and for replacing sugar-sweetened drinks with water. About 11.5 cups (2.7 liters) of fluids a day for women. HTMO@Wn% !TBB7wgvBPU73I.ht~z6#'SFF+!E6Mb_-C_:fTJU'ExSP~'X2B6:1n7>DgM*YXX$4fXL/zcb%6%g?,R The molar mass of this compound is equal to the molar mass of copper plus the molar mass of sulfur plus four times the molar mass of oxygen (since there are four oxygen atoms in the molecule). 4. 11.9 to be 38F (497.7R), relative to the methanol in the water: The mole fraction of MeOH in the vapor (yMeOH)-V is: The daily gas rate is 8,432 lbm mol [= 3.2 106 scf/(379.5 scf/lbm mol), where an scf is at 14.7 psia and 60F], so that the MeOH lost to the gas is 4.29 lbm mol (= 0.000509 8,432) or 137.3 lbm/D. m = number of moles of water.
Prediction of Conditions for Hydrate Formation in Natural Gases. 1945. The abscissa (x-axis) in each figure represents the lowest downstream pressure without hydrate formation, given the upstream pressure on the ordinate (y-axis) and the upstream temperature (a parameter on each line). 9M rBU b$|1*cltt->h ]na$;> ~u2m]viAD%$x d z (1996) "Sweat losses during various sports" Australian Journal of Nutrition and Dietetics 53(Suppl 4):1316, [5] Malisova O., Athanasatou A., Pepa A., Husemann M., Domnik K., Braun H., Mora-Rodriguez R., Ortega J.F., Fernandez-Elias V.E., Kapsokefalou M. (2016) "Water Intake and Hydration Indices in Healthy European Adults: The European Hydration Research Study (EHRS)" Nutrients 8(4):204, [6] Kant A. K., Graubard B. I. WebCamelBak has your hydration needs covered from kids water bottles and sports water bottles to travel mugs and drinkware for your car, home or classroom. At pressures above 6,000 psia, these gases will cool on expansion. 3. 1945. Constipation, characterized by slow gastrointestinal transit, small, hard stools, and difficulty in passing stool, has a number of causes including medication use, inadequate fiber intake, poor diet, and illness. Here is a video discussing how to find the empirical formula of copper sulfate. EFSA stands for European Food Safety Authority and IOM stands for the U.S. Institute of Medicine, data is based on reference 3. The formula for our hydrate is FeCl 3 6H 2 O. 63.55 + 32.06 + (4 x 16) = 159.61 grams per mole Divide the mass of your anhydrous (heated) salt sample by the molar mass of the anhydrous compound to get the number of moles of compound present. A body of studies examined by Popkin et. al [1] indicates that low to moderate dehydration may negatively affect cognitive performance. Once we do that, we will then divide the total mass of water by the mass of the hydrate. The difference in mass between the anhydrous and hydrated salt gives you the information you need to find the percentage of water in the hydrate.
How To Press Charges For False Cps Report Texas, Articles H