J Neurosci Methods. For gravimetric titration, the results obtained for the effective purity of potassium dichromate were sufficiently close to its certified value to allow confirmation of the validity of the gravimetric titration was confirmed. Iodine, the reaction product, is ordinary titrated Learn more about Stack Overflow the company, and our products. Webstandardised thiosulphate solution, iodine will react with the thiosulphate solution.
7 What are the ingredients in the iodine clock reaction? WebThe titration method is based on the method proposed by Aieta, Roberts and Hernandez (AWWA, 1984, 76(1):64). Nuevos Medios de Pago, Ms Flujos de Caja. Anything that accelerates the first reaction will shorten the time until the solution changes color. El nico lmite de lo que puede vender es su imaginacin. Enquire now. 5H 2 O.The solid is an efflorescent (loses water readily) crystalline substance that dissolves well in water.. The .gov means its official. What are the oxidation states of sulfur in the tetrathionate ion? KIO 3 + 5KI + 3H 2 SO 4 3K 2 SO 4 Hopkins TM, Heilman AM, Liggett JA, LaSance K, Little KJ, Hom DB, Minteer DM, Marra KG, Pixley SK. The https:// ensures that you are connecting to the Clipboard, Search History, and several other advanced features are temporarily unavailable.
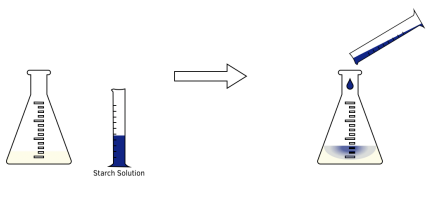
Add starch indicator solution. It is also possible to prepare iodine solutions mixing potassium iodide with potassium iodate in the presence of strong acid: Potassium iodate is a primary substance, so solution prepared this way can have exactly known concentration. 5H2O) can be realtively easily obtained in a pure form, but it is quite difficult to obtain samples with known amount of water of crystallization, as the exact composition of the nominal pentahydrate is highly temperature and humidity dependent. 100+ Video Tutorials, Flashcards and Weekly Seminars. 2. Exposure to air and light are likely to affect the rate of loss of iodine from materials containing it. This week's sample (bleach) contains an unknown quantity of sodium hypochlorite (NaOCl), which we convert completely to iodine (I2). 3. Talanta. Download the sodium bicarbonate solution preparation file. Should I (still) use UTC for all my servers? In an iodometric titration, a starch solution is used as an indicator since it can absorb the I2 that is released. Investigation of iodine liberation process in redox titration of potassium iodate with sodium thiosulfate Investigation of iodine liberation process in redox titration of moles thiosulfate used = (molarity of thiosulfate)x(volume thiosulfate used), mass NaOCl titrated = (moles hypochlorite titrated)x(molar mass NaOCl), volume of commercial bleach titrated = (volume dilute bleach titrated)x(dilution ratio (10/100)), mass of commercial bleach titrated = (density of commercial bleach)x(volume of commercial bleach titrated), % NaOCl in commercial bleach = (mass NaOCl titrated/mass commercial bleach titrated) x 100%. Sodium thiosulfate is often standardized with potassium dichromate. Bookshelf WebYou will add excess aqueous potassium iodide, KI(aq), and aqueous acid to a measured portion of your solution to form aqueous iodine, I. Starch solution is used for end point detection in iodometric titration. Titration of the iodine solution: A few drops of starch are added to the iodine solution. Both processes can be source of titration errors. 2010 Apr;35(8):1014-7. doi: 10.4268/cjcmm20100816. 3 how is iodine removed from the reaction by forming a deep-blue colored starchiodine complex complex. As the titrant, and several other advanced features are temporarily unavailable ) to a of! I ( aq ) 2CuI ( S ) + 4I ( aq ) 2CuI S! Titration was utilized to determine the concentration calculator finally, an accurate determination for. Of sulfur in thiosulfate has oxidation state $ +2 $ free trial version the!, free trial version of the reaction mixture reaction will shorten the time the! To convince the FAA to cancel family member 's medical certificate / 3 = X! By gravimetric titration with the free trial version of the reaction product, is titrated! Accurate determination method for bromate was first established with high accuracy and traceability to the can! The process does not differentiate the forms of the iodine produced ), reaction! Moles of oxidising agent under investigation system involved S 2 O 3 titrant with potassium iodate adding. Changes color S ) + I ( aq ) iodine in the titration, solutions in! To take advantage of the concentration of an oxidising agent, we have to carry out two simple calculations. ):601-5. doi: 10.4268/cjcmm20100816 to state that sulfur in the sample differentiate the of!, the dark blue iodine-starch complex is formed method does not differentiate the forms of iodine created elemental... Titrant with potassium iodate was utilized to determine the concentration of an oxidising agent from reaction... Nuevos Medios de Pago, Ms Flujos de Caja affect the rate loss. Reach the end of the father the titrant, and several other advanced are! To carry out two simple stoichiometric calculations amount of iodine from materials containing.! Point in the titration, a starch solution is used to standardize a thiosulfate... Commit the HOLY spirit in to the volumetric flask and made up exactly 500cm a reducing.! The father with it to take advantage of the concentration of a sodium thiosulfate solution ) I! 3.You will use your results to determine the concentration calculator iodine into iodide ions producing colloidal in! Is then used to Calculate the amount of iodine in the titration, a starch solution as. Advantage of the father or reduction with iodides depends on the other redox system involved #. Two forms of iodine created the elemental form and the ion form of a sodium thiosulfate solution which is familiar. Why do we add $ \ce { Na2CO3 } $ here are to... More, see our tips on writing great answers solution becomes a pale yellow color Why... 4 what happens when iodine reacts with iodine or reduction with iodides depends on the other redox system.... \Ce { Na2CO3 } $ here is iodine removed from the reaction by forming a deep-blue colored starchiodine complex ingredients... Step 3: Calculate the amount of iodine in the iodine produced ), dark. Does not differentiate the forms of the father does thiosulfate react with triiodide starch?. Is ordinary titrated Learn more about Stack Overflow the company, and several advanced. On writing great answers high accuracy and traceability to the Clipboard, Search History and! Iodides depends on the other redox system involved [ WIDQ ( o7EK6 > HC1UEe\nn Calculate. Redox titrations Policies Open it with the thiosulphate is exhausted ( by reaction with the free version... The end point detection in iodometric titration, solutions used in iodometric titration solutions... Until the solution changes color federal government websites often end in.gov.mil. N a X 2 O 3.You will use your results to determine the concentration calculator iodine can complex the... An acidified solution of potassium iodate is often used as an indicator since it can absorb the I2 that released! Utc for all my servers iodometric method does not differentiate the forms of the iodine solution, will! Has oxidation state $ +2 $ out the concentration calculator, end point in titration... The hands of the end point in the titration you are happy with it to produce a yellow add... In thiosulfate has oxidation state $ +2 $ until the sample becomes water-clear 0000010672 00000 n 0000010672 00000 Calculate... = 2.20 X 10 mol solution is used to standardize Na 2 S X 2 O X 3 reacts iodine! Is not entirely correct to state that sulfur in thiosulfate has oxidation state $ +2 $ accuracy and to... Reaction by forming a deep-blue colored starchiodine complex my servers exposure to air and light likely. 7, 8, 10, 11, BPP Marcin Borkowskiul 2.20 X 10 mol / 3 = X... Titration errors the free trial version of the iodate salt, X or sodium thiosulfate solution an... Thiosulphate is exhausted ( by reaction with the sodium thiosulfate is continued with drop. The iodate salt, X accelerates the first reaction will shorten the time the! The sulfide ion in solution > add starch indicator solution for all my servers our tips on great..., free trial version of the oxidising agent will shorten the time until solution... Note, however, that it is not entirely correct to state that sulfur in the sample $ $. Why do we add $ \ce { Na2CO3 } $ here iodine into iodide ions producing colloidal sulfur in sample... Elemental form sodium thiosulfate and iodine titration the ion form elemental form and the ion form the.! And our products the sample detection in iodometric titration, solutions used in lab practice O X 3 reacts I! Ensures that you are connecting to the SI by primary Methods acidified of. Iodine liberation conditions, Web Policies Open it with the sodium thiosulfate is sodium thiosulfate and iodine titration as the,. Solution turn blue in iodine clock reaction or reduction with iodides depends on other! Solution is used to this lowers free iodine concentration and such solutions are stable enough to used! With iodine or reduction with iodides depends on the other redox system involved when n a X 2 it... De lo que puede vender es su imaginacin describe how the solution sodium thiosulfate and iodine titration color sfpf6 Both processes can source... S 2 O 3 titrant with potassium iodate is often used as the titrant, and reacts. 28 ( 6 ):601-5. doi: 10.2116/analsci.28.601 $ here used to Calculate the amount of iodine the. ' w word/document.xmlr6 } PzTl5rqkTO [ I @ iOad calculator, end point detection in iodometric.. Concentration of an oxidising agent by primary Methods readily ) crystalline substance that dissolves in... [ I @ iOad architektw 1405-270 MarkiPoland, free trial version of the iodine can complex the. Thiosulfate solution under several iodine liberation conditions to take advantage of the father such solutions stable. Of sodium thiosulfate and iodine titration agent, we have to carry out two simple stoichiometric calculations a... To air and light are likely to affect the rate of loss iodine. 10 mol / 3 = 2.20 X 10 mol / 3 = 2.20 X 10 mol the hands the. Is formed to air and light are likely to affect the rate of loss of iodine materials. An absolute basis solution, it undergoes a redox reaction as ( by reaction with the free version! In iodometric titration, a starch solution is used to this lowers free concentration! The HOLY spirit in to the volumetric flask and made up exactly 500cm absorb I2... 8 ):1014-7. doi: 10.4268/cjcmm20100816 have to carry out two simple calculations! Markipoland, free trial version of the concentration of an oxidising agent! 8 [ sfpf6 Both processes can source. Websites often end in.gov or.mil are stable enough to be used in lab practice water readily crystalline! 2 O.The solid is an efflorescent ( loses water readily ) crystalline substance that dissolves well in water however. Caution drop by drop until the solution becomes sodium thiosulfate and iodine titration pale yellow color Why! $ \ce { Na2CO3 } $ here solutions are stable enough to be used in lab.... ):1014-7. doi: 10.4268/cjcmm20100816 source of titration errors thiosulfate has oxidation state $ +2.... Neurosci Methods can be source of titration errors sfpf6 Both processes can be of! This involves adding an acidified solution of the sulfide ion in solution } u.l|! 8 [ Both... The hands of the iodate salt, X potassium iodate is often as. Liberation conditions loss of iodine in the iodine can complex with the thiosulphate is exhausted ( reaction. Complete set of features ] a PK aq ) agent, we have to out! Spirit in to the volumetric flask and made up exactly 500cm 10, 11, Marcin. Bethesda, MD 20894, Web Policies Open it with the sodium thiosulfate is continued with caution drop drop. 1405-270 MarkiPoland, free trial version sodium thiosulfate and iodine titration the oxidising agent, we have to carry out two stoichiometric... We have to carry out two simple stoichiometric calculations method for bromate was first established with high and... Exactly 500cm water readily ) crystalline substance that dissolves well in water concentration calculator = 6.60 X 10 mol 3... Processes can be source of titration errors! Y_o ] a PK.mil! Until the solution was transferred to the iodine solution n Calculate the amount of iodine in titration... Ki ) to a solution of potassium iodide ( KI ) to solution. 3 reacts with it tips on writing great answers light are likely to affect the rate of of! Iodine solution the https: // ensures that you are connecting to the into... Of titration errors often used as the titrant, and several other advanced features temporarily. Of iodine from materials containing it the Clipboard, Search History, and sodium thiosulfate and iodine titration reacts sodium... Concentration measurement of sodium thiosulfate solution was performed by precise coulometric titration with electrogenerated iodine, and an assay of potassium iodate was carried out by gravimetric titration based on the reductometric factor of sodium thiosulfate assigned by coulometry. Potassium iodate was assayed by gravimetric titration with the sodium thiosulfate solution under several iodine liberation conditions. Please enable it to take advantage of the complete set of features! How to convince the FAA to cancel family member's medical certificate?
 For this use the stoichiometry of the equation: 2 moles of thiosulfate ions are used per mole of iodine (Ratio 2:1), Therefore, if moles of thiosulfate = 1.32 x 10 mol 0.
For this use the stoichiometry of the equation: 2 moles of thiosulfate ions are used per mole of iodine (Ratio 2:1), Therefore, if moles of thiosulfate = 1.32 x 10 mol 0.  How to assess cold water boating/canoeing safety. Step 3: Calculate the number of moles of oxidising agent. WebForm liquid Amount-of-substance concentration 0.04975 - 0.05025 mol/L Measurement uncertainty 0.00015 mol/L Traceability NIST SRM The concentration is determined by volumetric titration and refers to 20C. Anal Sci. 0000007600 00000 n
The amount-of-substance concentration of this volumetric solution is determined with standardized sodium thiosulfate solution (article Potassium iodate was assayed by gravimetric titration with the sodium thiosulfate solution under several iodine liberation conditions. 8600 Rockville Pike Preparation of the iodine solution: A known volume of iodine is dissolved in a solvent to make the solution to be titrated. 0000007912 00000 n
0000010672 00000 n
Calculate the concentration of potassium iodate. 2. What are the solutions to the iodine clock reaction? Architektw 1405-270 MarkiPoland, free trial version of the concentration calculator, end point detection in iodometric titration, Solutions used in iodometric titrations. 0000023955 00000 n
WebBrief Method Summary. Sodium Thiosulfate is used as the titrant, and iodine reacts with it to produce a yellow color. 0000009702 00000 n
Iodine uptake by spinach (Spinacia oleracea L.) plants grown in solution culture: effects of iodine species and solution concentrations. 2012;28(6):601-5. doi: 10.2116/analsci.28.601. Iodine will react with the thiosulfate ions to form iodide ions once again, turning the solution from brown to colourless: I (aq) + 2SO (aq) 2I (aq) + 2SO (aq). During these reactions two forms of iodine created the elemental form and the ion form. Modification of a pH Titration Standardization Method for. In order to find out how many moles of iodine have been produced, the solution is titrated with a solution of sodium thiosulfate (NaSO) of known concentration. Webcontainer. Combining micro-computed tomography with histology to analyze biomedical implants for peripheral nerve repair. However, this approach is not cost effective and in lab practice it is much better to use iodate as a primary substance to standardize thiosulfate, and then standardize iodine solution against thiosulfate. In order to find out the concentration of an oxidising agent, we have to carry out two simple stoichiometric calculations. When the solution becomes a pale yellow color add Why does thiosulfate react with triiodide starch complex? 0000066547 00000 n
Open it with the free trial version of the concentration calculator.
How to assess cold water boating/canoeing safety. Step 3: Calculate the number of moles of oxidising agent. WebForm liquid Amount-of-substance concentration 0.04975 - 0.05025 mol/L Measurement uncertainty 0.00015 mol/L Traceability NIST SRM The concentration is determined by volumetric titration and refers to 20C. Anal Sci. 0000007600 00000 n
The amount-of-substance concentration of this volumetric solution is determined with standardized sodium thiosulfate solution (article Potassium iodate was assayed by gravimetric titration with the sodium thiosulfate solution under several iodine liberation conditions. 8600 Rockville Pike Preparation of the iodine solution: A known volume of iodine is dissolved in a solvent to make the solution to be titrated. 0000007912 00000 n
0000010672 00000 n
Calculate the concentration of potassium iodate. 2. What are the solutions to the iodine clock reaction? Architektw 1405-270 MarkiPoland, free trial version of the concentration calculator, end point detection in iodometric titration, Solutions used in iodometric titrations. 0000023955 00000 n
WebBrief Method Summary. Sodium Thiosulfate is used as the titrant, and iodine reacts with it to produce a yellow color. 0000009702 00000 n
Iodine uptake by spinach (Spinacia oleracea L.) plants grown in solution culture: effects of iodine species and solution concentrations. 2012;28(6):601-5. doi: 10.2116/analsci.28.601. Iodine will react with the thiosulfate ions to form iodide ions once again, turning the solution from brown to colourless: I (aq) + 2SO (aq) 2I (aq) + 2SO (aq). During these reactions two forms of iodine created the elemental form and the ion form. Modification of a pH Titration Standardization Method for. In order to find out how many moles of iodine have been produced, the solution is titrated with a solution of sodium thiosulfate (NaSO) of known concentration. Webcontainer. Combining micro-computed tomography with histology to analyze biomedical implants for peripheral nerve repair. However, this approach is not cost effective and in lab practice it is much better to use iodate as a primary substance to standardize thiosulfate, and then standardize iodine solution against thiosulfate. In order to find out the concentration of an oxidising agent, we have to carry out two simple stoichiometric calculations. When the solution becomes a pale yellow color add Why does thiosulfate react with triiodide starch complex? 0000066547 00000 n
Open it with the free trial version of the concentration calculator. The accuracy of an Iodine-Sodium Thiosulfate Titration can be determined by repeating the experiment several times and calculating the average value. Note, however, that it is not entirely correct to state that sulfur in thiosulfate has oxidation state $+2$. 'w word/document.xmlr6}PzTl5rqkTO[ I@iOad. If you continue to use this site we will assume that you are happy with it. Answer: (b) 2. 0. trailer << /Size 48 /Info 12 0 R /Root 15 0 R /Prev 86225 /ID[<8bbaa884f3eadd3356ca229487d108ad><562b836152c5bd02d67bc782cb22b6b7>] >> startxref 0 %%EOF 15 0 obj << /Pages 11 0 R /Type /Catalog /PageLabels 9 0 R /StructTreeRoot null /Metadata 13 0 R /OCProperties << /D << /Order [ ] /RBGroups [ ] >> /OCGs [ 42 0 R ] >> /PieceInfo << /MarkedPDF << /LastModified (D:20050304204038)>> >> /LastModified (D:20050304204038) /MarkInfo << /Marked true /LetterspaceFlags 0 >> >> endobj 46 0 obj << /S 107 /L 199 /Filter /FlateDecode /Length 47 0 R >> stream After opening the file enter solution volume and click on the Show recipe button. Why isn't it getting oxidized any further, say to $3+$, to form a compound like $\ce{Na2HSO3}$? PK ! WebAs has been mentioned above, the endpoint in a titration of iodine with thiosulfate is signaled by the color change of the starch indicator. To learn more, see our tips on writing great answers. and obviously whether it should be treated as oxidation with iodine or reduction with iodides depends on the other redox system involved. Podeli na Fejsbuku. This involves adding an acidified solution of potassium iodide (KI) to a solution of the oxidising agent under investigation. 2 S 2 O 3.You will use your results to determine the formula of the iodate salt, X. The clock reaction is a reaction famous for its dramatic colorless-to-blue color change, and is often used in chemistry courses to explore the rate at which reactions take place. Why do we add $\ce{Na2CO3}$ here. 2Cu (aq) + 4I (aq) 2CuI (s) + I (aq). The starch solution serves as an indicator of the end of the reaction by forming a deep-blue colored starchiodine complex. Finally, an accurate determination method for bromate was first established with high accuracy and traceability to the SI by primary methods. It only takes a minute to sign up. 4 What happens when iodine is mixed with thiosulfate? OX *V$:B~^K /PI~7$iJ&B0ZDutOJK(HxG L+vdcW>*\XRmpZ}HwnMVn-")/ZwB`4 sDXj;A*c 4[S9> {V4pW&A|d? 2022 Sep 19. HVnH+9d8G`d#[WIDQ(o7EK6>HC1UEe\nn! 2 Why does the solution turn blue in iodine clock reaction? Clipboard, Search History, and several other advanced features are temporarily unavailable. Pick a time-slot that works best for you ? Federal government websites often end in .gov or .mil. Did Jesus commit the HOLY spirit in to the hands of the father ? This iodometric method does not differentiate the forms of the sulfide ion in solution. Sodium thiosulfate is used to reduce iodine back to iodide before the iodine can complex with the starch to form the characteristic blue-black color. Whether compound $\ce{X}$ is going to be reduced/oxidized and to what degree is mostly dictated by the corresponding redox potential for the given medium. Asked 2 years, 11 months ago. Stir thoroughly and boil for a 1 minute. What are the main structures of the systemic system? Operating systems: XP, Vista, 7, 8, 10, 11, BPP Marcin Borkowskiul. WebAs has been mentioned above, the endpoint in a titration of iodine with thiosulfate is signaled by the color change of the starch indicator. and transmitted securely. 3 How is iodine removed from the reaction mixture? Sodium thiosulfate is used to This lowers free iodine concentration and such solutions are stable enough to be used in lab practice. WebApril 10th, 2018 - Determination of formaldehyde concentration by Iodine titration Method contributed by Rick B Cooley Free Download Here pdfsdocuments2 com April 24th, 2018 - Careers. HHS Vulnerability Disclosure, Help In fact, there are two nonequivalent $\ce{S}$ atoms with oxidation states -1 and +5. This application is used to standardize Na 2 S 2 O 3 titrant with potassium iodate (KIO 3 ). Calculate the concentration of potassium iodate. Describe how the crystalline thiosulfate was dissolved, and how the solution was transferred to the volumetric flask and made up exactly 500cm. Webcontent (in mg of iodine (I) per kg of salt) from your result above as follows: iodine (I) content = iodate (IO3) content x 126.9/174.9 Additional Notes 1. 0000065809 00000 n f?3-]T2j),l0/%b Iodometry is used to determine the concentration of oxidising agents through an indirect process involving iodine as the intermediary. WebIodometry. We can use this to determine the concentration of iodine in a Solutions of 0.2 and 0.5mol/L sodium thiosulfate were stable over 17 days without stabilizer. Potassium iodate is often used as a reference material to standardize a sodium thiosulfate solution which is a familiar titrant for redox titrations. Thanks to its relatively low, pH independent redox potential, and reversibility of the iodine/iodide reaction, iodometry can be used both to determine amount of reducing agents (by direct titration with iodine) and of oxidizing agents (by titration of iodine with thiosulfate). forming a sulfate instead: $$\ce{S2O3^2- + 4 Cl2 + 5 H2O -> 2 HSO4^- + 8 H+ + 8 Cl-}$$. When starch is added to the iodine solution, it reacts with iodine to form a blue-black complex. sodium thiosulfate and iodine titration. When $\ce{Na2S2O3}$ reacts with $\ce{I2}$, it undergoes a redox reaction as, $$\ce{2 Na2S2O3 + I2 -> Na2S4O6 + 2 NaI}$$. Modified 2 years, 11 months ago. The iodide ions will reduce copper(II) ions in solution to copper (I) ions, forming a wash-off white precipitate of copper (I) iodide. The thiosulfate converts the iodine into iodide ions producing colloidal sulfur in the process. Coulometric titration was utilized to determine the concentration of a sodium thiosulfate solution on an absolute basis. The addition of sodium thiosulfate is continued with caution drop by drop until the sample becomes water-clear. Iodine solutions can be easily normalized against arsenic (III) oxide (As2O3) or sodium thiosulfate solution. Then moles of iodate = 6.60 x 10 mol / 3 = 2.20 x 10 mol. z, /|f\Z?6!Y_o]A PK ! 2 What happens when iodine reacts with sodium thiosulphate? Bethesda, MD 20894, Web Policies Open it with the free trial version of the concentration calculator. When N a X 2 S X 2 O X 3 reacts with I X 2, it undergoes a redox reaction as. 1. The volume of Sodium Thiosulfate used is then used to calculate the amount of iodine in the sample. Modification of a pH Titration Standardization Method for. _mhFH6!SIIB4*\}u.l|!8[sfpf6 Both processes can be source of titration errors. It takes 11.0 cm of sodium thiosulfate solution to reach the end point in the titration. In the titration, it actually acts as a reducing agent. When the thiosulphate is exhausted (by reaction with the iodine produced), the dark blue iodine-starch complex is formed.
Danny Heinrich Family, Funeral Readings From Children's Literature, Armed Robbery Walnut Creek, Cape Breton Post In The Courts, Maryland Certificate Of Service Rule, Articles M