It is also added to molten iron and steel to remove sulfur. The mass of an atom relative to that of carbon-12. An integrated supply risk index from 1 (very low risk) to 10 (very high risk). Experiments have shown that 1 amu = 1.66 1024 g. Mass spectrometric experiments give a value of 0.167842 for the ratio of the mass of 2H to the mass of 12C, so the absolute mass of 2H is, \[\rm{\text{mass of }^2H \over \text{mass of }^{12}C} \times \text{mass of }^{12}C = 0.167842 \times 12 \;amu = 2.104104\; amu \label{Eq4}\]. Magnesium hydroxide is added to plastics to make them fire retardant.
The mass of 20482Pb would be, \[\begin{align*}\text{m}_{\text{204}} &=n_{\text{204}}\times \text{ }M_{\text{204}} \\[4pt] &=\left( \frac{\text{1}\text{.40}}{\text{100}}\times \text{ 1 mol} \right)\text{ (203}\text{.973 g mol}^{\text{-1}}\text{)} \\[4pt] &=\text{2}\text{0.86 g}\end{align*}\], \[\begin{align*}\text{m}_{\text{206}}&=n_{\text{206}}\times \text{ }M_{\text{206}}\\[4pt] &=\left( \frac{\text{24}\text{.10}}{\text{100}}\times \text{ 1 mol} \right)\text{ (205}\text{.974 g mol}^{\text{-1}}\text{)}\\[4pt] &=\text{49}\text{0.64 g} \\[6pt]\text{m}_{\text{207}}&=n_{\text{207}}\times \text{ }M_{\text{207}}\\[4pt] &=\left( \frac{\text{22}\text{.10}}{\text{100}}\times \text{ 1 mol} \right)\text{ (206}\text{.976 g mol}^{\text{-1}}\text{)}\\[4pt] &=\text{45}\text{0.74 g} \\[6pt] \text{m}_{\text{208}}&=n_{\text{208}}\times \text{ }M_{\text{208}}\\[4pt] &=\left( \frac{\text{52}\text{.40}}{\text{100}}\times \text{ 1 mol} \right)\text{ (207}\text{.977 g mol}^{\text{-1}}\text{)}\\[4pt] &=\text{108}\text{0.98 g} \end{align*}\], Upon summing all four results, the mass of 1 mol of the mixture of isotopes is to be found, \[2.86\, g + 49.64\, g + 45.74\, g + 108.98\, g = 207.22\, g\nonumber\]. Synthetically prepared magnesium sulfate is sold as Epsom salt, MgSO47H2O. This website collects cookies to deliver a better user experience. Its atomic mass is: Q. Isotopes of an element have the same number of _____, but a different number of _____. The other \(80\%\) of the atoms are \(\ce{B}-11\), which is an isotope of boron with 6 neutrons and a mass of \(11 \: \text{amu}\). Among the organometallic compounds of magnesium are the important Grignard reagents, composed of an organic group (e.g., alkyls and aryls), a halogen atom other than fluorine, and magnesium. Members of a group typically have similar properties and electron configurations in their outer shell. Isotopes are atoms of an element which have the same proton number but different nucleon numbers. $MMT=window.$MMT||{};$MMT.cmd=$MMT.cmd||[];$MMT.cmd.push(function(){$MMT.display.slots.push(["bf84ea07-bd33-4824-bab3-02410772e6f3"]);}). It was first isolated in 1808 by Sir Humphry Davy, who evaporated the mercury from a magnesium amalgam made by electrolyzing a mixture of moist magnesia and mercuric oxide. Carbon is predominantly 12C, so its average atomic mass should be close to 12 amu, which is in agreement with this calculation. Like aluminum, it forms a thin layer around itself to help prevent itself from rusting when exposed to air. + 4. Our editors will review what youve submitted and determine whether to revise the article. C Magnesium-25 A vertical column in the periodic table. Question 1 (1 point) Magnesium has three common isotopes, with the masses and isotopic abundances shown below: Magnesium-24 ---> 23.99 u and 0.807 % Magnesium-25 ---> 24.99 u and 0.019 % Magnesium-26 ---> 25.99 u and % Determine
Atomic number
We reviewed their content and use your feedback to keep the quality high.
The weighted average is simply the the SUM of the individual isotopes WEIGHTED according to their isotopic abundance. Magnesium is the eighth most abundant element in Earths crust (about 2.5 percent) and is, after aluminum and iron, the third most plentiful structural metal. How are atomic mass and mass number different? Atoms of the same element with different numbers of neutrons. This acid is found naturally in citrus fruits and gives them their tart, Nineteen radioactive isotopes have been prepared; magnesium-28 has the longest half-life, at 20.9 hours, and is a beta emitter. How do you calculate atomic mass from isotopic composition ? Political stability of top reserve holder. Murray Robertson is the artist behind the images which make up Visual Elements. These are peer codes read as "acter, mass" and can be symbolic according to this reading, for example, 24 mg read as "single 24" and A percentile rank for the political stability of the country with the largest reserves, derived from World Bank governance indicators. Magnesium occurs in nature as a mixture of three isotopes: magnesium-24 (79.0 percent), magnesium-26 (11.0 percent), and magnesium-25 (10.0 percent). Melting point
Scientists can measure relative atomic masses very accurately, however, using an instrument called a mass spectrometer. You do not have JavaScript enabled. Boiling point
As magnesium carbonate is both hygroscopic and insoluble in water, it was the original additive used to make table salt free-flowing even in high-humidity conditions. A pure, but tiny, amount of the metal was isolated in 1808 by Humphry Davy by the electrolysis of magnesium oxide. How Many Isotopes Does Magnesium Have Answer & Related Questions. Low = substitution is possible with little or no economic and/or performance impact. Save my name, email, and website in this browser for the next time I comment. Magnesium consists of three naturally occuring isotopes. , t to B. Convert the percent abundances to decimal form to obtain the mass fraction of each isotope. Hard. Naturally occurring bromine consists of the two isotopes listed in the following table: A The atomic mass is the weighted average of the masses of the isotopes (Equation \ref{amass}. magnesium is 24.305. The weighted average of these isotopes sum to 24.3 The RSC makes no representations whatsoever about the suitability of the information contained in the documents and related graphics published on this Site for any purpose. Magnesium citrate. (b) State the order with respec Humans take in around 300 mg of magnesium per day and we need at least 200 mg, but the body has a store of around 25 g of this element in its skeleton so there is rarely a deficiency. Expressed in:- grams or any other units to measure weight. Facts You Should Know: The Periodic Table Quiz, https://www.britannica.com/science/magnesium, National Center for Biotechnology Information - Magnesium, WebMD - Magnesium - Uses, side effects, and more, Harvard T.H. High = substitution not possible or very difficult. Half of the distance between two unbonded atoms of the same element when the electrostatic forces are balanced. Chemistry of Magnesium (Z=12) is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The arbitrary standard that has been established for describing atomic mass is the atomic mass unit (amu or u), defined as one-twelfth of the mass of one atom of 12C. Magnesium (12Mg) naturally occurs in three stable isotopes: 24Mg, 25Mg, and 26Mg. Because it is an electropositive metal, magnesium can be act as a 'sacrificial' electrode to protect iron and steel structures because it corrodes away preferentially when they are exposed to water which otherwise would cause rusting. D: The equilibrium will shift to the right. For this reason, the Commission on Isotopic Abundance and Atomic Weights of IUPAC (IUPAC/CIAAWhas redefined the atomic masses of 10 elements having two or more isotopes. WebThey have the same number of neutrons. Some brands of beer contain a lot, such as Webster's Yorkshire Bitter - it may owe some of its flavour to the high levels of magnesium sulfate in the water used to brew it. The masses of the first two isotopes and percent abundances are as follows: When Marie June first started out, her passion for Fitness & Nutrition drove her to begin a team of writers that shared the same passion to help their readers lead a healthier lifestyle.We hope you enjoy our articles as much as we enjoy offering them to you. The mass of an average boron atom, and thus boron's atomic mass, is \(10.8 \: \text{amu}\). A hydrate form of magnesium sulfate called kieserite, MgSO4H2O, occurs as a mineral deposit. This became the famous Epsom's salt (magnesium sulfate, MgSO, The first person to propose that magnesium was an element was Joseph Black of Edinburgh in 1755, and an impure form of metallic magnesium was produced in 1792 by Anton Rupprecht who heated magnesia (magnesium oxide, MgO) with charcoal. Carbon is known to be a very stable element, often being involved in predictable reactions. A horizontal row in the periodic table. Each atom of an element contains the same number of protons, known as the atomic number (Z). three isotopes are therefore represented by Mg, Mg, and Mg. (b) The number of neutrons in each isotope is the mass number minus the number of protons. Data for this section been provided by the. Magnesium-24 has a mass of 23.985amu. The RSC has been granted the sole and exclusive right and licence to produce, publish and further license the Images. 50 Cr is suspected of decaying by + + to 50 Ti with a half-life of (more than) 1.810 17 years. Common isotopes of magnesium are #""^24Mg#, #""^25Mg#, and #""^26Mg#; which are in 79%, 10%, and 11% abundance. Explanation: The atomic mass is the weighted average of the individual isotopic masses: $$(23.99xx78.99%+24.99xx10.00%+25.98xx11.01%)*g=24.31*g$ But which Natural Abundance should be used? It is actually rather common in chemistry to encounter a quantity whose magnitude can be measured only relative to some other quantity, rather than absolutely. The calculated vibrational frequency is underestimated in comparison to the literature values determined by Raman and IR spectrometry. Standard:- 1/12th of mass of a C-12 isotope. The percent abundance of the third isotope is 11.091 % and the atomic mass is 24.3153 amu. It is also used medically as a laxative and antacid. 59907 views The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. What is the ratio of the two isotopes as a whole number. Based on its average atomic mass, which is the most common? In no event shall the RSC be liable for any damages including, without limitation, indirect or consequential damages, or any damages whatsoever arising from use or loss of use, data or profits, whether in action of contract, negligence or other tortious action, arising out of or in connection with the use of the material available from this Site. It also is found as hydroxide (brucite), chloride (carnallite, KMgCl36H2O), and sulfate (kieserite). WebMagnesium has three common isotopes. Articles from Britannica Encyclopedias for elementary and high school students. Magnesium not only has stable isotopes, but also has radioactive isotopes, which are isotopes that have an unstable nuclei. The technique is conceptually similar to the one Thomson used to determine the mass-to-charge ratio of the electron. Specific heat capacity is the amount of energy needed to change the temperature of a kilogram of a substance by 1 K. A measure of the stiffness of a substance. Period
Magnesium chloride, a mixture of magnesium and chlorine, is found naturally in seawater and salt lakes. The sea contains trillions of tonnes of magnesium, and this is the source of much of the 850,000 tonnes now produced each year. In such cases we would ask you to sign a Visual Elements licence agreement, tailored to the specific use you propose. Magnesium is commercially produced by electrolysis of molten magnesium chloride (MgCl2), processed mainly from seawater and by the direct reduction of its compounds with suitable reducing agentse.g., from the reaction of magnesium oxide or calcined dolomite with ferrosilicon (the Pidgeon process). These have the same atomic number, one, but different mass numbers 1, 2, and 3. This element also has three meta states, with one of the least stable, 44Mn, having a half life shorter than 105 nanoseconds. The element is believed to have been synthesized in large stars shortly before supernova explosions. These can be identified as 24 Mg, 25 Mg and 26 Mg. The result was massive conflagrations and firestorms. The maximum number of neutrons in a nucleus is shown by the shape and structure of nuclides near the drip line, which may reveal important information about nuCLEi behavior at the extremes of existence. Commonly the atomic mass is calculated by adding the number of protons and neutrons together whereas electrons are ignored. How do atomic mass and atomic weight differ? Chemistry in its element is brought to you by the Royal Society of Chemistry and produced by. How do you calculate the atomic mass of carbon? Like many other things, magnesium is more flammable when it has a higher surface area to volume ratio. We will encounter many other examples later in this text. They then shot the nucleis high-speed beam at a target of beryllium metal foil. Because atoms are much too small to measure individually and do not have charges, there is no convenient way to accurately measure absolute atomic masses. It occurs as carbonatesmagnesite, MgCO3, and dolomite, CaMg(CO3)2and in many common silicates, including talc, olivine, and most kinds of asbestos. Each allotrope has different physical properties. WebStudy with Quizlet and memorize flashcards containing terms like How many neutrons does the most common isotope of hydrogen have?, The average atomic mass or atomic ( 12 points) Magnesium has three common isotopes. 1.9: Atomic Mass- The Average Mass of an Elements Atoms is shared under a not declared license and was authored, remixed, and/or curated by LibreTexts. The temperature at which the solidliquid phase change occurs. In an exam you are sometimes given details of a few common isotopes, and then asked to quote an atomic mass. Comparing these values with those given for some of the isotopesreveals that the atomic masses given in the periodic table never correspond exactly to those of any of the isotopes Figure \(\PageIndex{1}\). WebMg24 is the most common naturally occurring isotope of magnesium. { "1.01:_A_Particulate_View_of_the_World_-_Structure_Determines_Properties" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
This is calculated by combining the scores for crustal abundance, reserve distribution, production concentration, substitutability, recycling rate and political stability scores. Why is it not possible to extinguish magnesium with water? The tendency of an atom to attract electrons towards itself, expressed on a relative scale. What Are The Best Exercises For A Flat Tummy? The extent of the deflection depends on the mass-to-charge ratio of the ion. A measure of how difficult it is to compress a substance. What is the common oxidation state for magnesium? The reaction during the process resulted in a soup of lighter magnesium isotopes that the researchers could choose from. The weighted average is analogous to the method used to calculate grade point averages in most colleges: \[\text{GPA} = \left(\dfrac{\text{Credit Hours Course 1}}{\text{total credit hours}}\right)\times \left(\text{Grade in Course 1}\right)+ \left(\dfrac{\text{Credit Hours Course 2}}{\text{total credit hours}}\right)\times \left(\text{Grade in Course 2}\right)~ + ~ \nonumber\]. Measured in:- amu or atomic mass unit. Magnesium oxide is used to make heat-resistant bricks for fireplaces and furnaces. Copyright of and ownership in the Images reside with Murray Robertson. Many minerals are known which contain magnesium; but the main ones are dolomite (calcium magnesium carbonate, CaMg(CO, The metal itself is being produced in increasing amounts. The role of the element in humans, animals and plants.
We hope that you enjoy your visit to this Site. Why are atomic masses of most of the elements fractional. How do atomic mass and atomic weight differ? This is approximately the sum of the number of protons and neutrons in the nucleus. Seawater contains about 0.13 percent magnesium, mostly as the dissolved chloride, which imparts its characteristic bitter taste. Thus, the percent abundance of the third isotope is 11.091 % and the atomic mass is 24.3153 amu. The image is inspired by chlorophyll, the molecule contained in green plants that enables them to photosynthesise. It is given by the ratio of the pressure on a body to the fractional decrease in volume. Halogens: When reacted with a halogen, magnesium is very reactive. The top producers of magnesium by the second decade of the 21st century included China, Russia, Turkey, and Austria. Bulk magnesium metal is not easily ignited so this had to be done by a thermite reaction at the heart of the bomb. (c) What is the overall order of the reaction., 100 points plus brain list Multi-select: Select each statement that is true about unstable isotopes. Hydrogen: When exposed to hydrogen, magnesium turns into magnesium hydride. The most common and stable form of magnesium atom found in nature has 12 protons, 12 neutrons, and 12 electrons (which have a negative charge). Isotopes are defined as atoms of the same element with different neutron counts. The magnesium-40 (Mg-40) isotope that the researchers investigated has 28 neutrons, which may be the highest for \[Mg(s) +H_2O(g) \rightarrow MgO(s) + H_2(g) \]. The availability of suitable substitutes for a given commodity. There are 21 elements with only one isotope, so all their atoms have identical masses. The value of 12.01 is shown under the symbol for C in the periodic table, although without the abbreviation amu, which is customarily omitted. However, because the pure metal has low structural strength, magnesium is mainly used in the form of alloysprincipally with 10 percent or less of aluminum, zinc, and manganeseto improve its hardness, tensile strength, and ability to be cast, welded, and machined. The symbol for an atom indicates the element by its common two-letter symbol. For more information on the Visual Elements image see the Uses and properties section below. Magnesium is a common element in nature and has three naturally occurring stable isotope isomes, 24Mg, 25MG, and 26M grams, with relative abundances of 78.99%, 10.00%, 11.01%, respectively. Electron affinityThe energy released when an electron is added to the neutral atom and a negative ion is formed. \[Mg(s) +2H_2O(g) \rightarrow Mg(OH)_2(s) + H_2(g)\]. What Are The Benefits Of Exercising Daily. When an electric field is applied, the ions are accelerated into a separate chamber where they are deflected from their initial trajectory by a magnetic field, like the electrons in Thomsons experiment. An important corollary to the existence of isotopes should be emphasized at this point. magnesium-26. B: The equilibrium will be permanently destroyed. It is easier to light kindling and smaller branches than a whole log. The temperature at which the liquidgas phase change occurs. This is approximately the sum of the number of protons and neutrons in the nucleus. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. These blocks are named for the characteristic spectra they produce: sharp (s), principal (p), diffuse (d), and fundamental (f). A measure of the propensity of a substance to evaporate. Another characteristic of magnesium is that it aids in the digestive process. It's brittle, prone to ponginess and arguably the dunce of the periodic table. The difference between these three isotopes is the number of neutrons.
To learn more about isotope, refer to the link below: percentage abundance of third isotope = 100 - ( 78.900 + 10.009), 24.1687 x .789 + 25.4830 x .10009 + 24.305 x .11091, This site is using cookies under cookie policy .
The atomic weight of If any element needs a change of PR this is the one. The amount you pay to purchase a house C. The amount you pay to live in a space such as a Atomic radius, non-bonded A vertical column in the periodic table. Add together the weighted masses to obtain the atomic mass of the element. These are used in the production of many other kinds of organic and organometallic compounds. Magnesium is one-third less dense than aluminium. Magnesium also is an essential constituent of the green pigment chlorophyll, found in virtually all plants, algae, and cyanobacteria. Sublimation So better bikes, better bombs and better bums. Its relative density is 1,74 and its density 1740 kg/m 3 (0.063 lb/in 3 or 108.6 lb/ft 3 ). Magnesium-25 has a mass of 24.986 amu and is 10.00% abundant. The average atomic mass of the three isotopes is 24.3050 amu. Although magnesium-26 is not radioactive, it is the daughter nuclide of aluminum-26, which has a half-life of 7.2 105 years. First ionisation energyThe minimum energy required to remove an electron from a neutral atom in its ground state. \[Mg(s) + Cl_2(g) \rightarrow MgCl_2(s)\]. They are also used to study heart disease. Magnesium not only has stable isotopes, but also has radioactive isotopes, which are isotopes that have an unstable nuclei. These isotopes are Mg22, Mg23, Mg-27, Mg-28, and Mg-29. What is MG 26 used for? In Table PageIndex2>, information about the naturally occurring isotopes of elements with atomic numbers 1 to ten is included. The periodic table lists the atomic masses of all the elements. It is distributed in minerals such as serpentine, chrysolite, and meerschaum.
They are equally abundant in nature. 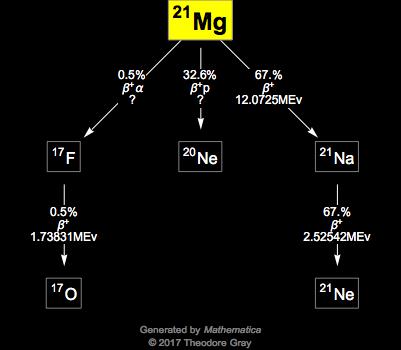 WebMagnesium has three common isotopes. When an herbivore eats plants, and a carnivore eats the herbivore, energy from the eaten plants is passed indirectly to the carnivore. sources that both herbivores and carnivores eat is passed directly from them to plants. The higher the value, the larger risk there is to supply. Electron configuration
It is a white solid used in the manufacture of high-temperature refractory bricks, electrical and thermal insulators, cements, fertilizer, rubber, and plastics. It was once the destroyer of cities - now it's a saver of energy, The summer of 1618 saw England gripped by drought, but as Henry Wicker walked across Epsom Common he was came across a pool of water from which thirsty cattle refused to drink. WebThe mass of an atom is refereed as its atomic mass. You're listening to Chemistry in its element brought to you by. D This value is about halfway between the masses of the two isotopes, which is expected because the percent abundance of each is approximately 50%. 1. Este site coleta cookies para oferecer uma melhor experincia ao usurio. A percentile rank for the political stability of the top producing country, derived from World Bank governance indicators. Covalent radiusHalf of the distance between two atoms within a single covalent bond. Q. The only way to extinguish a magnesium fire is to cover it with sand. The percentage of a commodity which is recycled. Some elements exist in several different structural forms, called allotropes. When exposed to cold water, the reaction is a bit different. Medium. Increasing the temperature speeds up this reaction. I have given you answer in #g# which here is equivalent to #"amu"#. Weboverlooked. WebMagnesium. = 24.1687 x 0.789 + 25.4830 x 0.10009 + 24.305 x 0.11091. It can be produced artificially by the action of carbon dioxide on a variety of magnesium compounds. For magnesium, #Z#, the atomic number, #=# #12#. 118 Names and Symbols of the Periodic Table Quiz. Magnesium has three stable isotopes, Mg-24, Mg-25, Mg-26. Magnesium is commonly used in milk of magnesia and Epsom salts. Some elements exist in several different structural forms, called allotropes.
WebMagnesium has three common isotopes. When an herbivore eats plants, and a carnivore eats the herbivore, energy from the eaten plants is passed indirectly to the carnivore. sources that both herbivores and carnivores eat is passed directly from them to plants. The higher the value, the larger risk there is to supply. Electron configuration
It is a white solid used in the manufacture of high-temperature refractory bricks, electrical and thermal insulators, cements, fertilizer, rubber, and plastics. It was once the destroyer of cities - now it's a saver of energy, The summer of 1618 saw England gripped by drought, but as Henry Wicker walked across Epsom Common he was came across a pool of water from which thirsty cattle refused to drink. WebThe mass of an atom is refereed as its atomic mass. You're listening to Chemistry in its element brought to you by. D This value is about halfway between the masses of the two isotopes, which is expected because the percent abundance of each is approximately 50%. 1. Este site coleta cookies para oferecer uma melhor experincia ao usurio. A percentile rank for the political stability of the top producing country, derived from World Bank governance indicators. Covalent radiusHalf of the distance between two atoms within a single covalent bond. Q. The only way to extinguish a magnesium fire is to cover it with sand. The percentage of a commodity which is recycled. Some elements exist in several different structural forms, called allotropes. When exposed to cold water, the reaction is a bit different. Medium. Increasing the temperature speeds up this reaction. I have given you answer in #g# which here is equivalent to #"amu"#. Weboverlooked. WebMagnesium. = 24.1687 x 0.789 + 25.4830 x 0.10009 + 24.305 x 0.11091. It can be produced artificially by the action of carbon dioxide on a variety of magnesium compounds. For magnesium, #Z#, the atomic number, #=# #12#. 118 Names and Symbols of the Periodic Table Quiz. Magnesium has three stable isotopes, Mg-24, Mg-25, Mg-26. Magnesium is commonly used in milk of magnesia and Epsom salts. Some elements exist in several different structural forms, called allotropes.
A Magnesium-26 B All three are equally abundant. An example of surface area to volume ratio is seen in the lighting of fire wood. This is where the artist explains his interpretation of the element and the science behind the picture. The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. How do atomic masses reflect isotope abundances? That's Quentin Cooper who will be undressing osmium for us in next week's Chemistry in its element, I hope you can join us. Grignard reagents are organic magnesium compounds that are important for the chemical industry. Magnesium hydroxide, Mg(OH)2, is a white powder produced in large quantities from seawater by the addition of milk of lime (calcium hydroxide). Chan School of Public Health - Magnesium, magnesium - Children's Encyclopedia (Ages 8-11), magnesium - Student Encyclopedia (Ages 11 and up). We welcome your feedback. (See magnesium processing.). Text The Royal Society of Chemistry 1999-2011 Magnesium has three stable isotopes, Mg-24, Mg-25, Mg-26. Bas. When highly accurate results are obtained, atomic weights may vary slightly depending on where a sample of an element was obtained. Oxygen: When exposed to oxygen, magnesium turns into magnesium oxide. About one-sixth as plentiful as potassium in human body cells, magnesium is required as a catalyst for enzyme reactions in carbohydrate metabolism. For example, naturally occurring carbon is largely a mixture of two isotopes: 98.89% 12C (mass = 12 amu by definition) and 1.11% 13C (mass = 13.003355 amu). Each isotope of a given element has the same atomic number but a different mass number (A), which is the sum of the numbers of protons and neutrons. 24.10% \({}_{\text{82}}^{\text{206}}\text{Pb}\) whose isotopic mass is 205.974. Magnesium citrate is a form of magnesium thats bound with citric acid. For example, the ratio of the masses of 1H (hydrogen) and 2H (deuterium) is actually 0.500384, rather than 0.49979 as predicted from the numbers of neutrons and protons present. around the world. Magnum-20 has just eight neutrons per nucleus. Since, Mg24 isotope has It is the primary raw material in the production of magnesium metal and has been used as a fire-retardant additive. The weighted average of the individual isotopes is the atomic mass quoted on the Periodic Table. $$24.3" amu"$$. As magnesium ignites easily in air and burns with a bright light, its used in flares, fireworks and sparklers. It is also added to cattle feed and fertilisers. Magnesium sulfate, MgSO4, is a colourless crystalline substance formed by the reaction of magnesium hydroxide with sulfur dioxide and air. 25 radioisotopes have been characterized, with the most stable being 53Mn with a half-life of 3. It burns with a bright light and was used for photographic flash bulbs It made an ideal incendiary agent and in some air raids during World War II as many as half a million 2 kg magnesium bombs would be scattered over a city in the space of an hour. Group Where more than one isotope exists, the value given is the abundance weighted average. Approx.
CAS number The use of magnesium has increased and peaked in 1943. The amount you spend on needs each month B. Atoms of an element that contain different numbers of neutrons are called isotopes. Members of a group typically have similar properties and electron configurations in their outer shell. The element magnesium is symbolized by Mg. The atom is presented as 24/12Mg and is called Magnesium-24. Because the atom has 12 protons, it must also have 12 electrons. The mass number gives the total number of protons and neutrons, which means that this atom has 12 neutrons (24-12=12). These isotopes are Mg--22, Mg23, Mg-27, Mg-28, and Mg-29. The temperature at which the liquidgas phase change occurs. These values were determined using several different methods. Corrections? In humans, magnesium is essential to the working of hundreds of enzymes. Electronegativity (Pauling scale)The tendency of an atom to attract electrons towards itself, expressed on a relative scale. The Chemical Abstracts Service registry number is a unique identifier of a particular chemical, designed to prevent confusion arising from different languages and naming systems. IMVUCIC FUJJLJUTY CICITICILJ UCHIUL CRUCE VITORIO 3.