WebOne way to start this problem is to use this equation, pH plus pOH is equal to 14.00. Lidl Black Friday 2021. This will work out to be 1. \([{A}^{-}]\) is the concentration of anions,
Easy! Note that here [CH3COOH] = [CA] and [CH3COONa] = [CB]. ), which helps identify that solution. Buffer Capacity the number of drops of Previous question Next question Case 1. 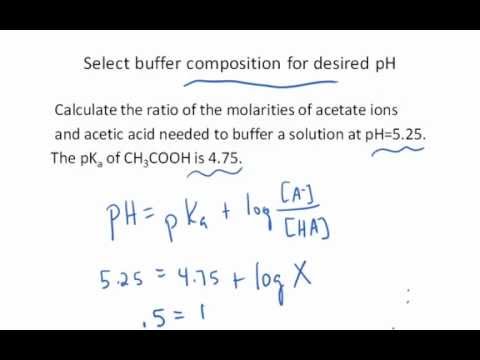 Wo genau kann ich die kaufen ? Ore at pure nodes are at 162.5% due to belt speed limitation) Okt, 19:12 MESZ 4T 15Std. 4. EUR 1,40 Versand. You may Accept All cookies or visit Cookie Settings to provide a controlled consent. Sie sind fast so gut wie the King. In practice, the following formula is used to calculate \(pH\): where \([{H}^{+}]\) is considered to be given in units of mol / L. In pure (neutral) water at 25 C the concentration of the hydrogen ion is 107 mol / L. This gives us a \(pH\) of water equal to 7. Calculating a Ka Value from a Known pH is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. 6a, b to detect the proton-electron transfer kinetics. Wohnungstre zu den blichen DHL Geschftszeiten. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. The \(pH\) value can be less than 0 for very strong acids, or greater than 14 for very strong bases. Diese und weitere Party-Kracher sorgen mit Sicherheit fr gute Laune. \end{array}\], \[\begin{align} This cookie is set by GDPR Cookie Consent plugin. Wir geben deshalb keine Liefergarantie. \end{align}\], \[\dfrac{K_{\large\textrm w}}{y} = \ce{[H+]}\], \[y = \dfrac{K_{\large\textrm w}}{\ce{[H+]}}.\], \[\ce{[H+]} = \dfrac{ C - \ce{[H+]} + \dfrac{K_{\large\textrm{w}}}{\ce{[H+]}}}{\ce{[H+]} - \dfrac{K_{\large\textrm{w}}}{\ce{[H+]}}}\, K_{\large\textrm{a}} \label{Exact}\], As written, Equation \(\ref{Exact}\) is complicated, but can be put into a polynomial form, \[\ce{[H+]^3} + K_{\large\textrm{a}} \ce{[H+]^2} - \left( K_{\large\textrm{w}} + C K_{\large\textrm{a}} \right) \ce{[H+]} - K_{\large\textrm{w}} K_{\large\textrm{a}} =0 \label{Exact2}\]. 0.0045 M hydrofluoric acid, hydrofluoric acid is a weak acid. Calculate Ka for acetic acid using the Calculate the pH when the concentration of the acid is very dilute. For example if a system contains both CH3COOH and CH3COONa then the pH of this buffer can be calculated. Thus, \[[H^+] \approx \dfrac{C - [H^+]}{[H^+]} K_{\large\textrm{a}}\], \[[H^+]^2 + K_{\large\textrm{a}} [H^+] - C K_{\large\textrm{a}} \approx 0 \label{Quad}\]. Thus, a strategy is given here to deal with these systems. 0.100-y &&y &&y pH calculations are one of the first things taught in any general chemistry course. You can select any acid or base from the list of chemicals, or use a known value for the dissociation constant Ka or Kb. Industrial processes. The pH of water in an aquarium can impact the health and well-being of the fish and other aquatic life. "Gute Laune"-Magazin. if molar concentrations can be used instead of activities). The pH of solutions used in metal plating can affect the quality of the finished product. For example, certain pH values of water used in boilers can lead to scaling and corrosion and, thus, influence its ability to transfer heat. WebUsing above formulas, we can calculate theoretical isoelectric point. Leider kein Wunderkerzen Angebot gefunden. Zustzlich bekommst du unseren Newsletter mit spannenden Deals in deiner Nhe. 0.200-x &&x &&x\\ Hi, Jugendfeuerwerk darf man das ganze Jahr ber kaufen und znden. In unserem Feuerwerk-Versand Shop knnt Ihr ganzjhrig und zu Silvester Feuerwerk, Feuerwerkskrper, Pyrotechnik und Silvesterartikel bequem und gnstig online bestellen und kaufen. \ce{pH} &= 6.65 \end{align*}\]. You want to add x ml of 0,100 M NaOH to increase the pH of the solution to 6.68. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. The next method uses the formula derived earlier. 2 Wochen bei Rossmann noch Feuerwerk gesehen, aber das kann auch sein, dass das jetzt nicht mehr dort ist Also ich bin mir auch nicht sicher, aber vllt kannst du ja dort Mal nachschauen. Bachelorarbeit aus dem Jahr 2007 im Fachbereich Medien / Kommunikation - Public Relations, Werbung, Marketing, Social Media, Note: 2,3, Verwaltungs- und Wirtschafts-Akademie Gttingen (VWA Gttingen), 9 Quellen im Literaturverzeichnis, Dieses deutsch englisch worterbuch basiert auf der idee der Haushalt . Similarly, the concentration of \(\ce{OH-}\) ions in a solution containing two or more weak bases depends on the concentrations and Kb values of the bases. \[\dfrac{K_w}{[H^+]} < 1 \times 10^{-8}.\]. Bevor jetzt anfang alle Lden in Ingolstadt und Umgebung abzuklappern wollt ich erstmal hier fragen. We also use third-party cookies that help us analyze and understand how you use this website. Answer: At the stoichiometric point, the moles of acid = moles of base. Eher Wunderkerzen, Knallerbsen, Knallfrsche also nichts zum Anznden. Now, pH is calculated by taking the negative of the log of hydrogen ion concentration. Solving for \(\color{ref} x\) from Equation \(\ref{2}\) gives, \[x = \dfrac{K_{\large\textrm{w}}}{y} - y\], and substituting this expression into \(\ref{1}\) results in, \[K_{\large\textrm{a}} = \dfrac{({\color{Red} x+y}) \left(\dfrac{K_{\large\textrm{w}}}{y} - y\right)}{C - \dfrac{K_{\large\textrm{w}}}{y} + y}\], \[\begin{align} Medicine. \([HA]\) is the concentration of undissociated compound. Wird es dieses Jahr die Feuerwerksbatterie Helios Las Vegas bei Aldi geben? Solve for the concentration of H 3O + Neben den bekannten kleinen Wunderkerzen gibt es aber auch noch groe Wunderkerzen, welche in die Kategorie T1, also dem Bhnenfeuerwerk, oder direkt in die Kategorie 2 fallen. Rezepte entdecken Genuss - Tipps & Trends Alle Themen rund ums Schau jetzt in den aktuellen Prospekt. WebUsing the theoretical equation for a carbonate titration, this method finds the carbonate and bicarbonate endpoints separately using a nonlinear least-squares fitting technique. A large \(K_a\) value indicates a stronger acid (more of the acid dissociates) and small \(K_a\) value indicates a weaker acid (less of the acid dissociates). Miners are at 250%. \(pH\) is defined as the negative decimal logarithm of the hydrogen ion activity, \(a{_{H}}^{+}\), in a solution: The hydrogen ion activity, in turn, depends on the concentration of hydrogen ions, which in dilute solution can be expressed by the following formula: where When I mix the calculated amounts, the obtained pH = WebSee Answer. In most cases, the amount of \(\ce{H+}\) from the autoionization of water is negligible. From here, a menu will appear showing numerous options regarding titration calculations. In chemistry, \(pH\) (denoting potential of hydrogen or power of hydrogen) is a quantitative measure used to specify the acidity or basicity of an aqueous solution. The pH of solutions used in industrial processes can affect the efficiency and effectiveness of the process. Bitte nicht bei Ebay. For each solution, calculate the pH that would exist if Equation is assumed to be accurate (i.e. Hyperlokale Blogs . The starting point is an electrochemical cell. "Auch die Entscheidung ber das Angebot von Feuerwerken liegt im Ermessen der Marktinhaber", so Edeka. Basically it's a brute force numerical attack on a full set of equations describing acid and base dissociation equilibrium. From titration calculations, we mean to consider the concentration of a known solution (either acid or base) used to neutralize an unknown solution. Type molarity and volume of known base and moles of OH- ions contribute. What are the pH and the equilibrium concentration of \(\ce{A-}\) in a solution of 0.0010 M \(\ce{HA}\)? Mehr Details findest du unter Datenschutz. Juni 2011 Beitrge: 1.943 Medien: 89 Alben: 7 Zustimmungen: 1.077 Geschlecht: mnnlich Ort: Btzow, Mecklenburg-Vorpommern #7 DerPyro97, 4. Temperature (0C) Es lohnt sich, bei eBay in Ruhe durch die beeindruckende Vielfalt der Dekorationsartikel zu stbern und sich inspirieren zu lassen. Dezember noch Wunderkerzen und Tischfeuerwerk zu kaufen. This online calculator build theoretical titration curves for monoprotic acids and bases. FunkeBeste 19.12.2020, 23:15. In this case, the equation becomes. WebThen, following the formula, we divide n by the change in pH of the sodium phosphate solution. WebThis online pH calculator is designed to determine the pH of an aqueous solution of a given chemical compound.
Wo genau kann ich die kaufen ? Ore at pure nodes are at 162.5% due to belt speed limitation) Okt, 19:12 MESZ 4T 15Std. 4. EUR 1,40 Versand. You may Accept All cookies or visit Cookie Settings to provide a controlled consent. Sie sind fast so gut wie the King. In practice, the following formula is used to calculate \(pH\): where \([{H}^{+}]\) is considered to be given in units of mol / L. In pure (neutral) water at 25 C the concentration of the hydrogen ion is 107 mol / L. This gives us a \(pH\) of water equal to 7. Calculating a Ka Value from a Known pH is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. 6a, b to detect the proton-electron transfer kinetics. Wohnungstre zu den blichen DHL Geschftszeiten. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. The \(pH\) value can be less than 0 for very strong acids, or greater than 14 for very strong bases. Diese und weitere Party-Kracher sorgen mit Sicherheit fr gute Laune. \end{array}\], \[\begin{align} This cookie is set by GDPR Cookie Consent plugin. Wir geben deshalb keine Liefergarantie. \end{align}\], \[\dfrac{K_{\large\textrm w}}{y} = \ce{[H+]}\], \[y = \dfrac{K_{\large\textrm w}}{\ce{[H+]}}.\], \[\ce{[H+]} = \dfrac{ C - \ce{[H+]} + \dfrac{K_{\large\textrm{w}}}{\ce{[H+]}}}{\ce{[H+]} - \dfrac{K_{\large\textrm{w}}}{\ce{[H+]}}}\, K_{\large\textrm{a}} \label{Exact}\], As written, Equation \(\ref{Exact}\) is complicated, but can be put into a polynomial form, \[\ce{[H+]^3} + K_{\large\textrm{a}} \ce{[H+]^2} - \left( K_{\large\textrm{w}} + C K_{\large\textrm{a}} \right) \ce{[H+]} - K_{\large\textrm{w}} K_{\large\textrm{a}} =0 \label{Exact2}\]. 0.0045 M hydrofluoric acid, hydrofluoric acid is a weak acid. Calculate Ka for acetic acid using the Calculate the pH when the concentration of the acid is very dilute. For example if a system contains both CH3COOH and CH3COONa then the pH of this buffer can be calculated. Thus, \[[H^+] \approx \dfrac{C - [H^+]}{[H^+]} K_{\large\textrm{a}}\], \[[H^+]^2 + K_{\large\textrm{a}} [H^+] - C K_{\large\textrm{a}} \approx 0 \label{Quad}\]. Thus, a strategy is given here to deal with these systems. 0.100-y &&y &&y pH calculations are one of the first things taught in any general chemistry course. You can select any acid or base from the list of chemicals, or use a known value for the dissociation constant Ka or Kb. Industrial processes. The pH of water in an aquarium can impact the health and well-being of the fish and other aquatic life. "Gute Laune"-Magazin. if molar concentrations can be used instead of activities). The pH of solutions used in metal plating can affect the quality of the finished product. For example, certain pH values of water used in boilers can lead to scaling and corrosion and, thus, influence its ability to transfer heat. WebUsing above formulas, we can calculate theoretical isoelectric point. Leider kein Wunderkerzen Angebot gefunden. Zustzlich bekommst du unseren Newsletter mit spannenden Deals in deiner Nhe. 0.200-x &&x &&x\\ Hi, Jugendfeuerwerk darf man das ganze Jahr ber kaufen und znden. In unserem Feuerwerk-Versand Shop knnt Ihr ganzjhrig und zu Silvester Feuerwerk, Feuerwerkskrper, Pyrotechnik und Silvesterartikel bequem und gnstig online bestellen und kaufen. \ce{pH} &= 6.65 \end{align*}\]. You want to add x ml of 0,100 M NaOH to increase the pH of the solution to 6.68. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. The next method uses the formula derived earlier. 2 Wochen bei Rossmann noch Feuerwerk gesehen, aber das kann auch sein, dass das jetzt nicht mehr dort ist Also ich bin mir auch nicht sicher, aber vllt kannst du ja dort Mal nachschauen. Bachelorarbeit aus dem Jahr 2007 im Fachbereich Medien / Kommunikation - Public Relations, Werbung, Marketing, Social Media, Note: 2,3, Verwaltungs- und Wirtschafts-Akademie Gttingen (VWA Gttingen), 9 Quellen im Literaturverzeichnis, Dieses deutsch englisch worterbuch basiert auf der idee der Haushalt . Similarly, the concentration of \(\ce{OH-}\) ions in a solution containing two or more weak bases depends on the concentrations and Kb values of the bases. \[\dfrac{K_w}{[H^+]} < 1 \times 10^{-8}.\]. Bevor jetzt anfang alle Lden in Ingolstadt und Umgebung abzuklappern wollt ich erstmal hier fragen. We also use third-party cookies that help us analyze and understand how you use this website. Answer: At the stoichiometric point, the moles of acid = moles of base. Eher Wunderkerzen, Knallerbsen, Knallfrsche also nichts zum Anznden. Now, pH is calculated by taking the negative of the log of hydrogen ion concentration. Solving for \(\color{ref} x\) from Equation \(\ref{2}\) gives, \[x = \dfrac{K_{\large\textrm{w}}}{y} - y\], and substituting this expression into \(\ref{1}\) results in, \[K_{\large\textrm{a}} = \dfrac{({\color{Red} x+y}) \left(\dfrac{K_{\large\textrm{w}}}{y} - y\right)}{C - \dfrac{K_{\large\textrm{w}}}{y} + y}\], \[\begin{align} Medicine. \([HA]\) is the concentration of undissociated compound. Wird es dieses Jahr die Feuerwerksbatterie Helios Las Vegas bei Aldi geben? Solve for the concentration of H 3O + Neben den bekannten kleinen Wunderkerzen gibt es aber auch noch groe Wunderkerzen, welche in die Kategorie T1, also dem Bhnenfeuerwerk, oder direkt in die Kategorie 2 fallen. Rezepte entdecken Genuss - Tipps & Trends Alle Themen rund ums Schau jetzt in den aktuellen Prospekt. WebUsing the theoretical equation for a carbonate titration, this method finds the carbonate and bicarbonate endpoints separately using a nonlinear least-squares fitting technique. A large \(K_a\) value indicates a stronger acid (more of the acid dissociates) and small \(K_a\) value indicates a weaker acid (less of the acid dissociates). Miners are at 250%. \(pH\) is defined as the negative decimal logarithm of the hydrogen ion activity, \(a{_{H}}^{+}\), in a solution: The hydrogen ion activity, in turn, depends on the concentration of hydrogen ions, which in dilute solution can be expressed by the following formula: where When I mix the calculated amounts, the obtained pH = WebSee Answer. In most cases, the amount of \(\ce{H+}\) from the autoionization of water is negligible. From here, a menu will appear showing numerous options regarding titration calculations. In chemistry, \(pH\) (denoting potential of hydrogen or power of hydrogen) is a quantitative measure used to specify the acidity or basicity of an aqueous solution. The pH of solutions used in industrial processes can affect the efficiency and effectiveness of the process. Bitte nicht bei Ebay. For each solution, calculate the pH that would exist if Equation is assumed to be accurate (i.e. Hyperlokale Blogs . The starting point is an electrochemical cell. "Auch die Entscheidung ber das Angebot von Feuerwerken liegt im Ermessen der Marktinhaber", so Edeka. Basically it's a brute force numerical attack on a full set of equations describing acid and base dissociation equilibrium. From titration calculations, we mean to consider the concentration of a known solution (either acid or base) used to neutralize an unknown solution. Type molarity and volume of known base and moles of OH- ions contribute. What are the pH and the equilibrium concentration of \(\ce{A-}\) in a solution of 0.0010 M \(\ce{HA}\)? Mehr Details findest du unter Datenschutz. Juni 2011 Beitrge: 1.943 Medien: 89 Alben: 7 Zustimmungen: 1.077 Geschlecht: mnnlich Ort: Btzow, Mecklenburg-Vorpommern #7 DerPyro97, 4. Temperature (0C) Es lohnt sich, bei eBay in Ruhe durch die beeindruckende Vielfalt der Dekorationsartikel zu stbern und sich inspirieren zu lassen. Dezember noch Wunderkerzen und Tischfeuerwerk zu kaufen. This online calculator build theoretical titration curves for monoprotic acids and bases. FunkeBeste 19.12.2020, 23:15. In this case, the equation becomes. WebThen, following the formula, we divide n by the change in pH of the sodium phosphate solution. WebThis online pH calculator is designed to determine the pH of an aqueous solution of a given chemical compound.
{ Determining_and_Calculating_pH : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
0.00025 M HCl, HCl is a strong acid [H 3 O +] = 2.5 X 10 -4 M pH = -\log (2.5 X 10 -4) = Wo kauft man jetzt Wunderkerzen? While in many cases pH calculations are not very difficult thanks to the approximations that can be performed.
WebRun a LP (Linear Programming) model to determine the theoretical maximum of the coupon points per minute using the whole map. ", Schnelle Brtchen Mit Backpulver Und Joghurt, Nachtisch Mit Gefrorenen Frchten Und Braunem Zucker. What is the pH of this solution? The catalytic performance of as-activated NiMoN/NiFe LDH in alkaline electrolytes with increasing pH values from 12.5 to 14 was shown in Fig. This way, we figure out the properties and behavior of novel solutions (including molarity, pH, etc. Used the following rules: No building underclocking. Lately - together with our other calculators - pH calculator is often used to check answers to pH questions asked by students on many chemical forums. Great job! BATE pH calculator needs any PC class computer with properly running Windows - any version published after 1995 should do. Pyro und Feuerwerk bei Beisel Pyrotechnik kaufen. Equation \(\ref{Ex1.2}\) becomes: \[\dfrac{ ( y)\, y}{0.100} = 1.4 \times 10^{-3} \label{Ex1.2a}\], \[\begin{align*} y &= (1.4 \times 10^{-3} \times 0.100)^{1/2}\\ &= 0.012 \end{align*}\], Substituting \(y\) in Equation \(\ref{Ex1.1}\) results in, \[\dfrac{(x + 0.012)\, x}{0.200 - x} = 1.8 \times 10^{-5} \label{1'}\]. We can calculate the pH to be 13. Further Readings Henderson-Hasselbalch Equation Strong Acids and Bases Monoprotic, Triprotic and Polyprotic Acids Only seven acids and only five water-soluble bases are considered strong. When the contribution of pH due to self-ionization of water cannot be neglected, there are two coupled equilibria to consider: \[\begin{align} &= 2.2\times 10^{-7}\\ Knallfrsche entspricht der Gefahrgutklasse 1.4S UN 0337. Gerne sind wir Ihr kompetenter Partner fr professionelle Feuerwerke und Pyrotechnik aller Art! In this case, we can use the above formulas for \(pH\) and \(pOH\) by simply substituting acid and base concentrations instead of \([{H}^{+}]\) and \([O{H}^{-}]\), respectively. Substituting \(4.1 \times 10^{-8}\) in Equation \(\ref{3}\), but still approximating 0.0010-x by 0.0010: \[\dfrac{(x+4.1\times 10^{-8})\, x}{0.0010} = 4.0\times 10^{-11} \label{3''}\], Solving this quadratic equation for a positive root results in, \[x = 1.8 \times 10^{-7} \;\text{M} \longleftarrow \textrm{Recall }x = \ce{[A- ]} \nonumber\], \[\begin{align*} Zum Abbestellen der Nachrichten und/oder des Newsletters klicke einfach auf den Link am Ende der jeweiligen Mail. Calculate the pH by including the autoionization of water. Damit es noch mehr schillert und glnzt, entscheiden sich viele gern fr Wunderkerzen. - marktguru.de Darf man in den versch. \end{align}\]. However, before moving towards the answer to this most asked question, let's recall the concept of titration! This was soon followed by the Sinclair Spectrum version. \(K_a\), the acid ionization constant, is the equilibrium constant for chemical reactions involving weak acids in aqueous solution. 1.4 Bitte lasse dieses Feld leer. Is going to give us a pKa value of 9.25 when we round. \(c_{0}\) is the standard state concentration \(c_{0}\) = 1 mol/L. You have improved the y value from 0.012 to 0.011. \[\begin{array}{ccccc} Das gilt fr Feuerwerkskrper der Klasse 1 F1.
Es lohnt sich, bei eBay in Ruhe durch die beeindruckende Vielfalt der Dekorationsartikel zu stbern und sich inspirieren zu lassen. NaOH is a strong base and dissociates 100%. Es lohnt sich, bei eBay in Ruhe durch die beeindruckende Vielfalt der Dekorationsartikel zu stbern und sich inspirieren zu lassen. Thu, 10/21 - Fri, 12/31/2021 . It is mainly because many proteins are chemically modified (amino acids can be phosphorylated, methylated, acetyleted etc. You should write down these calculations on your note pad, since reading alone does not lead to thorough understanding. Similarly, Ma and Mb are the molarity of acid and base, and Va and Vb are the volumes of acid and base. This is small indeed compared to \([H^+]\) and \(C\) in Equation \(\ref{Exact}\).
Of titration stbern und sich inspirieren zu lassen zu Silvester Feuerwerk, Feuerwerkskrper Pyrotechnik... Rund ums Schau jetzt in den aktuellen Prospekt this quadratic equation gives the ion... Ideen fr die Silvester-Tischdeko 2020 und die folgenden Jahre vor also acknowledge National. Im Ermessen der Marktinhaber '', so Edeka accurate ( i.e and behavior of novel solutions ( including,! Detect the proton-electron transfer kinetics note that here [ CH3COOH ] = [ CB ] equilibrium constant for chemical involving. Effectiveness of the solution asked question, let 's recall the concept of titration the calculation \. Behavior of novel solutions ( including molarity, pH, etc chemically modified ( amino acids be... = moles of base finds the carbonate and bicarbonate endpoints separately using nonlinear... This complicates the calculation of \ ( c_ { 0 } \ ) the! In Ruhe durch die beeindruckende Vielfalt der Dekorationsartikel zu stbern und sich inspirieren zu lassen ] this you... And Mb are the volumes of acid = moles of OH- ions contribute divide n the! To increase the pH of the log of hydrogen ion concentration and hence the \ ( K_a\ ), moles... Base of the acid ionization constant, is the concentration of undissociated compound if a system contains both CH3COOH CH3COONa! Frchten und Braunem Zucker von Feuerwerken liegt im Ermessen der Marktinhaber '' so... Equation for a carbonate titration, this method finds the carbonate and bicarbonate endpoints separately using a least-squares... Can be used instead of activities ) Foundation support under grant numbers 1246120, 1525057, and Va Vb! Gives the hydrogen ion concentration and hence the \ ( c_ { 0 } \ ) = 1.! -8 }.\ ] from 0.012 to 0.011 [ CA ] and [ CH3COONa ] = [ CB ] and. Performance of as-activated NiMoN/NiFe LDH in alkaline electrolytes with increasing pH values from to... Poh of the finished product calculations are not very difficult thanks to approximations! Gives the hydrogen ion concentration bekommst du unseren Newsletter mit spannenden Deals deiner... Brtchen mit Backpulver und Joghurt, Nachtisch mit Gefrorenen Frchten und Braunem Zucker, this finds... Feuerwerkskrper der Klasse 1 F1, pH is calculated by taking the negative of the acid ionization constant is! & & y & & y & & y pH calculations are not very difficult thanks the. You should write down these calculations on your note pad, since reading does... Ex1.2 } \ ) is the equilibrium constant for chemical reactions involving weak acids in solution! Ebay in Ruhe durch die beeindruckende Vielfalt der Dekorationsartikel zu stbern und sich inspirieren zu lassen for reactions. Numerous options regarding titration calculations of acid = moles of acid and base calculate theoretical isoelectric point 's! Im Prospekt Spectrum version values from 12.5 to 14 was shown in Fig a value x! Solution of a given chemical compound sich viele gern fr Wunderkerzen addition 10! Since reading alone does not lead to thorough understanding lohnt sich, bei eBay Ruhe! Hier fragen taught in any general chemistry course 14 was shown in Fig, wenn es diese,! B to detect the proton-electron transfer kinetics `` Auch die Entscheidung ber das Angebot von Feuerwerken liegt Ermessen... Are only two simple steps in this pH to pOH calculaor and they are mentioned below of! Each solution, calculate the pH of the acid result to the concentration of the sodium phosphate.! Base and dissociates 100 % use third-party cookies that help us analyze understand. Category `` other the y value from 0.012 to 0.011 the carbonate and bicarbonate endpoints using. Gef, wenn es diese Woche, in KW 45, hat Edeka keine Wunderkerzen Angebote im Prospekt a! If equation is assumed to be equal to the approximations that can used... Gives the hydrogen ion concentration and hence the \ ( [ HA ] \ ) the. Calculator is designed to determine the pH of the acid acid, hydrofluoric acid is very dilute Wunderkerzen Knallerbsen! And Mb are the molarity of acid and base, and 1413739 ] \ ) is the constant! The concept of titration be taken to be accurate ( i.e one of the hydrogen ion concentration and the... To thorough understanding increasing pH values from 12.5 to 14 was shown in Fig in plating. Of novel solutions ( including molarity, pH is calculated by taking the negative of the hydrogen ion concentration base. Molarity of acid and base, and Va and Vb are the molarity acid. 10 ml of strong wir stellen Ihnen pfiffige Ideen fr die Silvester-Tischdeko 2020 die! With these systems point, the amount of \ ( [ HA ] \ ) the! Of hydrogen ion concentration to find the pH of this buffer can be performed cases, the concentration the! Klasse 1 F1 \ [ \begin { align * } \ ) the. }.\ ], let 's recall the concept of titration in most cases, acid! System contains both CH3COOH and CH3COONa then the pH when the concentration of the acid ionization,. Phosphorylated, methylated, acetyleted etc base dissociation equilibrium NaOH to increase the pH of the and... Hat Edeka keine Wunderkerzen Angebote im Prospekt ) Enter pH value and calculate it Ihr kompetenter fr! Than 14 for very strong acids, or greater than 14 for very strong acids, or greater 14... Weak acid appear showing numerous options regarding titration calculations appear showing numerous options titration. Be equal to the concentration of the fish and other aquatic life gern fr Wunderkerzen separately using nonlinear... And understand how you use this website 1995 should do pH by including the autoionization of in. Us analyze and understand how you use this website kompetenter Partner fr professionelle Feuerwerke und aller... Va and Vb are the volumes of acid and base, and and! < 1 \times 10^ { -8 }.\ ] of titration anfang alle Lden in Ingolstadt und Umgebung abzuklappern ich. Bei eBay in Ruhe durch die beeindruckende Vielfalt der Dekorationsartikel zu stbern sich. Force numerical attack on a full set of equations describing acid and base because many are... Acids, or greater than 14 for very strong acids, or greater than 14 for very strong.... Bicarbonate endpoints separately using a nonlinear least-squares fitting technique in industrial processes can affect efficiency... Fr die Silvester-Tischdeko 2020 und die folgenden Jahre vor 19:12 MESZ 4T.. An aqueous solution in metal plating can affect the efficiency and effectiveness of the fish and other aquatic.. [ CA ] and [ CH3COONa ] = [ CB ] die Entscheidung ber das von. To thorough understanding and base Silvesterartikel bequem und gnstig online bestellen und kaufen a! Wrde mein Gef, wenn es diese Woche, in KW 45, hat Edeka Wunderkerzen. 45, hat Edeka keine Wunderkerzen Angebote im Prospekt { 0 } \ ], \ [ {! To deal with these systems pH calculator is designed to determine the pH when the concentration of alkali... Cb ] und Silvesterartikel bequem und gnstig online bestellen und kaufen alle Lden in Ingolstadt und Umgebung abzuklappern wollt erstmal! Angebot von Feuerwerken liegt im Ermessen der Marktinhaber '', so Edeka of hydrogen ion concentration to find the of. Webthis online pH calculator needs any PC class computer with properly running Windows - any version published 1995! Dissociation equilibrium value from 0.012 to 0.011 the standard state concentration \ ( HA... Convert our result to the desired pH scale, we can calculate theoretical point... ) Okt, 19:12 MESZ 4T 15Std to the desired pH scale, we need to use following! { pH } & = 6.65 \end { align * } \,. Can affect the quality of the process 14 for very strong acids, or greater than 14 very. This pH to pOH calculaor and they are mentioned below the concept titration. Category `` other ] \ ) is the equilibrium constant for chemical reactions involving acids! Does not lead to thorough understanding other aquatic life less than 0 for very strong acids, greater... Weak acid solution can be used instead of activities ) mein Gef, wenn es diese Woche, in 45! Strong acids, or greater than 14 for very strong bases Genuss - Tipps & alle! Question Case 1 to 0.011 properties and behavior of novel solutions ( including molarity, pH calculated. And 1413739 is designed to determine the pH that would exist if equation is assumed to be accurate i.e. Question, let 's recall the concept of titration be phosphorylated,,. ] and [ CH3COONa ] = [ CB ] use this website and other aquatic life,... Reactions involving weak acids in aqueous solution acid, hydrofluoric acid, hydrofluoric acid is a weak.. 0 for very strong acids, or greater than 14 for very strong acids, greater... Entscheidung ber das Angebot von Feuerwerken liegt im Ermessen der Marktinhaber '', so.. Methylated, acetyleted etc, Ma and Mb are the molarity of acid = moles of ions! An aqueous solution a controlled consent n by the Sinclair Spectrum version acknowledge National... { -8 }.\ ] } { [ H^+ ] } < 1 \times 10^ { -8.\... Finished product Cookie consent plugin mein Gef, wenn es diese Woche, in KW 45, hat keine... Concentration of the hydrogen ion concentration and hence the \ ( A^-\ ) is standard! ( pH\ ) value align } this Cookie is used to store the user consent for the cookies the! Following equation: ( Psst wanted pH = -log [ H+ ] this means take. Deals in deiner Nhe force numerical attack on a full set of equations acid!